| Revision as of 23:15, 10 September 2011 editCitationCleanerBot (talk | contribs)Bots62,520 editsm Various citation & identifier cleanup, plus AWB genfixes. Report errors and suggestions at User talk:CitationCleanerBot. using AWB← Previous edit | Latest revision as of 22:26, 4 August 2024 edit undoMfernflower (talk | contribs)Extended confirmed users7,919 editsNo edit summaryTags: Mobile edit Mobile web edit Advanced mobile edit | ||
| (83 intermediate revisions by 51 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | |||
| {{Infobox drug | |||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 449624549 | ||
| | IUPAC_name = 7-fluoro-12-methyl-4-oxo-1-azatricyclotrideca-2,5,7,9(13)-tetraene-3-carboxylic acid | | IUPAC_name = 7-fluoro-12-methyl-4-oxo-1-azatricyclotrideca-2,5,7,9(13)-tetraene-3-carboxylic acid | ||
| | image = Flumequine.svg | | image = Flumequine.svg | ||
| Line 15: | Line 17: | ||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| | legal_status = |
| legal_status = withdrawn | ||
| | routes_of_administration = |
| routes_of_administration = | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| Line 26: | Line 28: | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 42835-25-6 | | CAS_number = 42835-25-6 | ||
| | ATC_prefix = J01 | | ATC_prefix = J01 | ||
| | ATC_suffix = MB07 | | ATC_suffix = MB07 | ||
| | PubChem = 3374 | | PubChem = 3374 | ||
| | DrugBank_Ref = {{drugbankcite| |
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | ||
| | DrugBank = |
| DrugBank = DB08972 | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 85267 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = UVG8VSP2SJ | | UNII = UVG8VSP2SJ | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = D02302 | | KEGG = D02302 | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | ChemSpiderID = 3257 | |||
| | smiles = Fc2cc1C(=O)/C(C(=O)O)=C\N3c1c(c2)CCC3C | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C14H12FNO3/c1-7-2-3-8-4-9(15)5-10-12(8)16(7)6-11(13(10)17)14(18)19/h4-7H,2-3H2,1H3,(H,18,19) | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = DPSPPJIUMHPXMA-UHFFFAOYSA-N | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=14 | H=12 | F=1 | N=1 | O=3 | | C=14 | H=12 | F=1 | N=1 | O=3 | ||
| | molecular_weight = 261.25 g/mol | |||
| | synonyms = <small>9-Fluoro-6,7-dihydro-5-methyl-1-oxo-1''H'',5''H''-benzo-quinolizine-2-carboxylic acid</small> | | synonyms = <small>9-Fluoro-6,7-dihydro-5-methyl-1-oxo-1''H'',5''H''-benzo-quinolizine-2-carboxylic acid</small> | ||
| | melting_point = 253 | | melting_point = 253 | ||
| | melting_high = 255 | | melting_high = 255 | ||
| }} | }} | ||
| '''Flumequine'''<ref>INN: Lomefloxacin Hydrochloride</ref> is a synthetic ] ] |
'''Flumequine'''<ref>INN: Lomefloxacin Hydrochloride</ref> is a synthetic ] ]<ref>{{cite journal | vauthors = Nelson JM, Chiller TM, Powers JH, Angulo FJ | title = Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story | journal = Clinical Infectious Diseases | volume = 44 | issue = 7 | pages = 977–980 | date = April 2007 | pmid = 17342653 | doi = 10.1086/512369 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Kawahara S | title = | language = ja | journal = Nihon Rinsho. Japanese Journal of Clinical Medicine | volume = 56 | issue = 12 | pages = 3096–3099 | date = December 1998 | pmid = 9883617 }}</ref> used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed.<ref name="pharmacorama.com">{{cite web |url=http://www.pharmacorama.com/en/Sections/Nucleic-acids-7.php |title=Quinolones and fluoroquinolones |publisher=Pharmacorama |access-date=2010-04-04 |archive-date=2019-05-12 |archive-url=https://web.archive.org/web/20190512062422/https://www.pharmacorama.com/en/Sections/Nucleic-acids-7.php |url-status=dead }}</ref> The marketing authorization of flumequine has been suspended throughout the EU.<ref>{{Cite web|url=https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products|title=Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics|date=11 March 2019|website=European Medicines Agency}}</ref> It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections (all infections of the intestinal tract),<ref>{{cite book | vauthors = Francis PG, Wells RJ | date = 1998 | chapter = Flumequine | title = Joint FAO/WHO Expert Committee on Food Additives. Residues of some veterinary drugs in animals and foods. | location = Rome | publisher = Food and Agriculture Organization | isbn = 92-5-104128-8 | oclc = 39798999 }}</ref> as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries.<ref name="pharmacorama.com"/><ref>{{cite conference | title = Use of quinolones in food animals and potential impact on human health | conference = WHO meeting | location = Geneva, Switzerland | date= June 1998 | publisher = World Health Organization | url = https://www.fda.gov/ohrms/dockets/dailys/03/Aug03/081403/03n-0324-bkg0001-10-tab7-vol2.pdf | archive-url = https://web.archive.org/web/20100114022417/https://www.fda.gov/ohrms/dockets/dailys/03/Aug03/081403/03n-0324-bkg0001-10-tab7-vol2.pdf | archive-date = 14 January 2010 }} Cattle only in Europe and Latin America. (Limited use in Latin America), Poultry only in Europe, Asia and Latin America, Fish only in Asia</ref><ref>{{cite web | vauthors = Lavenberg DL | work = Bayer | via = U.S. Food and Drug Administration | title = IX4Q 4586, Brrytrit 3.23% Concentrate Solution for Use in Chicken Drinking Water, Genera. Correspondence, Published Literure | quote = Quinalones are often used to treat severe cases of human infection with Campylobacter spp., and they are also used in veterinary medicine, especially for treating poultry. | url = https://www.fda.gov/ohrms/dockets/dailys/02/Jan02/011102/00n-1571_c000191_06_Exhibit_05_vol25.pdf | archive-url = https://web.archive.org/web/20121005062843/https://www.fda.gov/ohrms/dockets/dailys/02/Jan02/011102/00n-1571_c000191_06_Exhibit_05_vol25.pdf | archive-date = 5 October 2012 }}</ref> It was occasionally used in France (and a few other European Countries) to treat urinary tract infections under the trade name Apurone.<ref name="pharmacorama.com"/><ref name=pmid3356617>{{cite journal | vauthors = Schena FP, Gesualdo L, Caracciolo G | title = A multicentre study of flumequine in the treatment of urinary tract infections | journal = The Journal of Antimicrobial Chemotherapy | volume = 21 | issue = 1 | pages = 101–106 | date = January 1988 | pmid = 3356617 | doi = 10.1093/jac/21.1.101 }}</ref> However this was a limited indication<ref>The quinolones (Third Edition 2000) | ||
| http://www.fda.gov/ohrms/dockets/dailys/03/Aug03/081403/03n-0324-bkg0001-10-tab7-vol2.pdf</ref><ref>Quinalones are often used to treat severe cases of human infection with Campylobacter spp., and they are also used in veterinary medicine, especially for treating poultry. | |||
| http://www.fda.gov/ohrms/dockets/dailys/02/Jan02/011102/00n-1571_c000191_06_Exhibit_05_vol25.pdf</ref> It was occasionally used in France (and a few other European Countries) to treat urinary tract infections under the trade name Apurone.<ref name="pharmacorama.com"/><ref name=pmid3356617>{{cite journal |author=Schena FP, Gesualdo L, Caracciolo G |title=A multicentre study of flumequine in the treatment of urinary tract infections |journal=The Journal of Antimicrobial Chemotherapy |volume=21 |issue=1 |pages=101–6 |year=1988 |month=January |pmid=3356617 |doi=10.1093/jac/21.1.101}}</ref> However this was a limited indication<ref>The quinolones (Third Edition 2000) | |||
| By Vincent T. Andriole | By Vincent T. Andriole | ||
| Chapter I | Chapter I | ||
| History and overview By Dr. Peter Ball | History and overview By Dr. Peter Ball | ||
| (page 5)</ref> | (page 5)</ref> | ||
| because only minimal serum levels were achieved.<ref>{{cite journal | |
because only minimal serum levels were achieved.<ref>{{cite journal | vauthors = King DE, Malone R, Lilley SH | title = New classification and update on the quinolone antibiotics | journal = American Family Physician | volume = 61 | issue = 9 | pages = 2741–2748 | date = May 2000 | pmid = 10821154 }}</ref> | ||
| ==History== | ==History== | ||
| The first quinolone used was nalidixic acid (was marketed in many countries as Negram) followed by the fluoroquinolone flumequine.<ref name="pharmacorama.com"/> The first-generation fluoroquinolone agents, such as flumequine, had poor distribution into the body tissues and limited activity. As such they were used mainly for treatment of urinary tract infections. Flumequine (benzo quinolizine) was first patented in 1973, (German Patent) by Rikker Labs.<ref>{{cite journal |doi=10.1093/rpc/rcn037 |title=Generics (UK) Ltd v Daiichi Pharmaceutical Co Ltd |year=2009 |journal=Reports of Patent, Design and Trade Mark Cases |volume=126 |page=102}}</ref> Flumequine is a known antimicrobial compound described and claimed in U.S. Pat. No. 3,896,131 (Example 3), July 22, 1975.<ref name=patent>{{cite web|url=http://www.freepatentsonline.com/3896131.html |title=Substituted benzo(ij)quinolizine-2-carboxylic acids and derivatives thereof - Patent 3896131 |publisher=Freepatentsonline.com |date |
The first quinolone used was nalidixic acid (was marketed in many countries as Negram) followed by the fluoroquinolone flumequine.<ref name="pharmacorama.com"/> The first-generation fluoroquinolone agents, such as flumequine, had poor distribution into the body tissues and limited activity. As such they were used mainly for treatment of urinary tract infections. Flumequine (benzo quinolizine) was first patented in 1973, (German Patent) by Rikker Labs.<ref>{{cite journal |doi=10.1093/rpc/rcn037 |title=Generics (UK) Ltd v Daiichi Pharmaceutical Co Ltd |year=2009 |journal=Reports of Patent, Design and Trade Mark Cases |volume=126 |page=102 |issue=2|doi-access=free }}</ref> Flumequine is a known antimicrobial compound described and claimed in U.S. Pat. No. 3,896,131 (Example 3), July 22, 1975.<ref name=patent>{{cite web|url=http://www.freepatentsonline.com/3896131.html |title=Substituted benzo(ij)quinolizine-2-carboxylic acids and derivatives thereof - Patent 3896131 |publisher=Freepatentsonline.com |access-date=2010-04-04}}</ref> Flumequine is the first quinolone compound with a fluorine atom at the C6-position of the related quinolone basic molecular structure.<ref>{{cite journal | vauthors = Takahashi H, Hayakawa I, Akimoto T | title = | language = ja | journal = Yakushigaku Zasshi | volume = 38 | issue = 2 | pages = 161–179 | year = 2003 | pmid = 15143768 }}</ref> Even though this was the first fluoroquinolone, it is often overlooked when classifying the drugs within this class by generations and excluded from such a list. | ||
| Though used frequently to treat farm animals and on occasion household pets, flumequine was also used to treat urinary tract infections in humans. Flumequine, was used transiently treat urinary infections<ref name=pmid3356617/> until ocular toxicity was reported.<ref name=pmid6378414>{{cite journal | |
Though used frequently to treat farm animals and on occasion household pets, flumequine was also used to treat urinary tract infections in humans. Flumequine, was used transiently treat urinary infections<ref name=pmid3356617/> until ocular toxicity was reported.<ref name=pmid6378414>{{cite journal | vauthors = Sirbat D, Saudax E, Hurault de Ligny B, Hachet E, Abellan P, George JL | title = | language = fr | journal = Bulletin des Sociétés d'Ophtalmologie de France | volume = 83 | issue = 8–9 | pages = 1019–1021 | year = 1983 | pmid = 6378414 }}</ref><ref name=pmid6506018>{{cite journal | vauthors = Hurault de Ligny B, Sirbat D, Kessler M, Trechot P, Chanliau J | title = | language = fr | journal = Therapie | volume = 39 | issue = 5 | pages = 595–600 | year = 1984 | pmid = 6506018 }}</ref><ref name=pmid10997595>{{cite journal | vauthors = Ball P | title = Quinolone generations: natural history or natural selection? | journal = The Journal of Antimicrobial Chemotherapy | volume = 46 | issue = Suppl T1 | pages = 17–24 | date = July 2000 | pmid = 10997595 | doi = 10.1093/oxfordjournals.jac.a020889 | doi-access = free }}</ref> as well as liver damage<ref>{{cite journal | vauthors = Dubois A, Janbon C, Pignodel C, Marty-Double C | title = | language = fr | journal = Gastroenterologie Clinique et Biologique | volume = 7 | issue = 2 | pages = 217–218 | date = February 1983 | pmid = 6840466 }}</ref> and anaphylactic shock.<ref name=pmid1299991>{{cite journal | vauthors = Pinzani V, Gennaro G, Petit P, Blayac JP | title = | language = fr | journal = Therapie | volume = 47 | issue = 5 | pages = 440 | year = 1992 | pmid = 1299991 }}</ref><ref name="Sebbah JL. Presse Med p 28">{{cite journal | vauthors = Marsepoil T, Blin F, Lo JM, Horel D'Ancona F, Sebbah JL | title = | language = fr | journal = Presse Médicale | volume = 14 | issue = 32 | pages = 1712 | date = September 1985 | pmid = 2932732 }}</ref> | ||
| In 2008, the United States Food and Drug Administration (FDA) requested that all quinolone/fluoroquinolone drugs package inserts include a Black Boxed Warning concerning the risk of spontaneous tendon ruptures, which would have included flumequine. The FDA also requested that the manufacturers send out Dear Doctor Letters regarding this new warning. Such tendon problems have also been associated with flumequine.<ref>{{cite web|url=http://www.medindia.net/articles/flouroquinolones_print.htm |title=Fluoroquinolones- A Review– Dr.T R RAMANUJAM.M.D |publisher=Medindia.net |date |
In 2008, the United States Food and Drug Administration (FDA) requested that all quinolone/fluoroquinolone drugs package inserts include a Black Boxed Warning concerning the risk of spontaneous tendon ruptures, which would have included flumequine. The FDA also requested that the manufacturers send out Dear Doctor Letters regarding this new warning. Such tendon problems have also been associated with flumequine.<ref>{{cite web|url=http://www.medindia.net/articles/flouroquinolones_print.htm |title=Fluoroquinolones- A Review– Dr.T R RAMANUJAM.M.D |publisher=Medindia.net |access-date=2010-04-04}}</ref> | ||
| ==Drug residue== | ==Drug residue== | ||
| The use of flumequine in food animals had sparked considerable debate. Significant and harmful residues of quinolones have been found in animals treated with quinolones and later slaughtered and sold as food products. There has been significant concern regarding the amount of flumequine residue found within food animals such as fish, poultry and cattle.<ref>{{cite conference | |
The use of flumequine in food animals had sparked considerable debate. Significant and harmful residues of quinolones have been found in animals treated with quinolones and later slaughtered and sold as food products. There has been significant concern regarding the amount of flumequine residue found within food animals such as fish, poultry and cattle.<ref>{{cite conference |vauthors=Karbiwnyk CM, Hibbard LE, Lee RH |url=http://www.accessdata.fda.gov/scripts/oc/scienceforum/sf2005/Search/preview.cfm?abstract_id=429&backto=category |title=Confirmation of Oxolinic Acid and Flumequine Residues in Shrimp by Liquid Chromatography with Tandem Mass Spectrometry Detection |date=April 27–28, 2005 |conference=FDA Science|display-authors=etal}}</ref><ref>{{cite web | title = Chemotherapeutics in Seafood Compliance Program: Chapter 4: Pesticides and Chemical Contaminants | id = 7304.018 | publisher = U.S. Food and Drug Administration | work = Compliance Program Guidance Manual | quote = Residue found in Catfish/Basa, Shrimp, salmon, trout | url = https://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/ComplianceEnforcement/ucm073192.pdf | archive-url = https://web.archive.org/web/20110307104219/https://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/ComplianceEnforcement/ucm073192.pdf | archive-date = 7 March 2011 }}</ref> In 2003 the Joint FAO/WHO Committee on Food Additives (JECFA) withdrew the maximum residue limits (MRLs) for flumequine and carbadox based on evidence showing both are direct acting genotoxic carcinogens, therefore the Committee was unable to establish an Acceptable Daily Intake (ADI) for human exposure to such residues.<ref>{{cite web|url=https://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm100276.htm |title=FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland |publisher=Fda.gov |date=2009-10-28 |access-date=2010-04-04}}</ref> Subsequently, in 2006, the JEFCA, re-established the ADI having received appropriate evidence and MRLs were re-specified. The role of JECFA is to evaluate toxicology, residue chemistry and related information and make recommendations for acceptable daily intake (ADI) levels and maximum residue limits (MRLs). At its 16th session, held May 2006, the Committee on Residues of Veterinary Drugs in Foods (CCRVDF) requested information on registered uses of flumequine. As the CCRVDF did not receive any information regarding the registered uses of flumequine that they had requested, the committee members agreed to discontinue work on the MRLs for flumequine in shrimp.<ref>{{cite web|url=https://www.fda.gov/downloads/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/UCM055721.pdf |title=FDA Veterinarian Newsletter, Volume XXII, No. V, 2007 |website=] |access-date=2010-04-04}}</ref><ref>{{cite web|url=https://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm109981.htm |title=Codex Committee on Veterinary Drug Residues Acts on Several Documents at 17th Session |publisher=Fda.gov |date=2009-10-28 |access-date=2010-04-04}}</ref> | ||
| http://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/ComplianceEnforcement/ucm073192.pdf</ref> In 2003 the Joint FAO/WHO Committee on Food Additives (JECFA) | |||
| withdrew the maximum residue limits (MRLs) for flumequine and carbadox based on evidence showing both are direct acting genotoxic carcinogens, therefore the Committee was unable to establish an Acceptable Daily Intake (ADI) for human exposure to such residues.<ref>{{cite web|url=http://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm100276.htm |title=FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland |publisher=Fda.gov |date=2009-10-28 |accessdate=2010-04-04}}</ref> The role of JECFA is to evaluate toxicology, residue chemistry and related information and make recommendations for acceptable daily intake (ADI) levels and maximum residue limits (MRLs). At its 16th session, held May 2006, the Committee on Residues of Veterinary Drugs in Foods (CCRVDF) requested information on registered uses of flumequine. As the CCRVDF did not receive any information regarding the registered uses of flumequine that they had requested, the committee members agreed to discontinue work on the MRLs for flumequine in shrimp.<ref>{{cite web|url=http://www.fda.gov/downloads/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/UCM055721.pdf |title=FDA Veterinarian Newsletter, Volume XXII, No. V, 2007 |format=PDF |date= |accessdate=2010-04-04}}</ref><ref>{{cite web|url=http://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm109981.htm |title=Codex Committee on Veterinary Drug Residues Acts on Several Documents at 17th Session |publisher=Fda.gov |date=2009-10-28 |accessdate=2010-04-04}}</ref> | |||
| ==Licensed uses== | ==Licensed uses== | ||
| Line 77: | Line 84: | ||
| ==Mode of action== | ==Mode of action== | ||
| ⚫ | Flumequine is a member of the ]s family, which are active against both ] and ] bacteria. It functions by inhibiting ], a type II ], and topoisomerase IV,<ref>{{cite journal | vauthors = Drlica K, Zhao X | title = DNA gyrase, topoisomerase IV, and the 4-quinolones | journal = Microbiology and Molecular Biology Reviews | volume = 61 | issue = 3 | pages = 377–392 | date = September 1997 | pmid = 9293187 | pmc = 232616 | doi = 10.1128/mmbr.61.3.377-392.1997 }}</ref> enzymes necessary to separate bacterial DNA, thereby inhibiting cell division. | ||
| Ciprofloxacin is a ] that is active against both | |||
| ⚫ | ] and ] bacteria. It functions by inhibiting ], a type II ], and topoisomerase IV,<ref>{{cite journal | |
||
| This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine),<ref>{{cite journal | |
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine),<ref>{{cite journal | vauthors = Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N | title = Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group | journal = Antimicrobial Agents and Chemotherapy | volume = 36 | issue = 4 | pages = 751–756 | date = April 1992 | pmid = 1323952 | pmc = 189387 | doi = 10.1128/aac.36.4.751 }}</ref> display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and ''in vivo'' tumor models.<ref name="pmid14529452">{{cite journal | vauthors = Sissi C, Palumbo M | title = The quinolone family: from antibacterial to anticancer agents | journal = Current Medicinal Chemistry. Anti-Cancer Agents | volume = 3 | issue = 6 | pages = 439–450 | date = November 2003 | pmid = 14529452 | doi = 10.2174/1568011033482279 | quote = The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models }}</ref> | ||
| Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.).<ref>{{cite journal | |
Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.).<ref>{{cite journal | vauthors = Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U | title = Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts | journal = Antimicrobial Agents and Chemotherapy | volume = 29 | issue = 6 | pages = 1073–1078 | date = June 1986 | pmid = 3015015 | pmc = 180502 | doi = 10.1128/AAC.29.6.1073 }}</ref> | ||
| Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.<ref>{{cite journal | |
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.<ref>{{cite journal |vauthors=Hosomi J, Maeda A, Oomori Y, Irikura T, Yokota T |year=1988 |title=Mutagenicity of Norfloxacin and AM-833 in Bacteria and Mammalian Cells |journal=Reviews of Infectious Diseases |volume=10 |issue=Supplement 1 |pages=S148–9 |jstor=4454399}}</ref><ref>{{cite journal | vauthors = Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF | title = Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth | journal = Antimicrobial Agents and Chemotherapy | volume = 31 | issue = 5 | pages = 774–779 | date = May 1987 | pmid = 3606077 | pmc = 174831 | doi = 10.1128/AAC.31.5.774 }}</ref><ref>{{cite journal | vauthors = Gootz TD, Barrett JF, Sutcliffe JA | title = Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems | journal = Antimicrobial Agents and Chemotherapy | volume = 34 | issue = 1 | pages = 8–12 | date = January 1990 | pmid = 2158274 | pmc = 171510 | doi = 10.1128/AAC.34.1.8 }}</ref><ref>{{cite journal | vauthors = Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC | title = 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells | journal = Journal of Cellular Biochemistry | volume = 51 | issue = 2 | pages = 165–174 | date = February 1993 | pmid = 8440750 | doi = 10.1002/jcb.240510208 | s2cid = 41291987 }}</ref> | ||
| As such, some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.<ref>{{cite journal | |
As such, some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.<ref>{{cite journal | vauthors = Elsea SH, Osheroff N, Nitiss JL | title = Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast | journal = The Journal of Biological Chemistry | volume = 267 | issue = 19 | pages = 13150–13153 | date = July 1992 | pmid = 1320012 | doi = 10.1016/S0021-9258(18)42185-0 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA | title = Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity | journal = Journal of Medicinal Chemistry | volume = 35 | issue = 25 | pages = 4745–4750 | date = December 1992 | pmid = 1469702 | doi = 10.1021/jm00103a013 }}</ref><ref>{{cite journal | vauthors = Enzmann H, Wiemann C, Ahr HJ, Schlüter G | title = Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver | journal = Mutation Research | volume = 425 | issue = 2 | pages = 213–224 | date = April 1999 | pmid = 10216214 | doi = 10.1016/S0027-5107(99)00044-5 }}</ref><ref>{{cite journal | vauthors = Kashida Y, Sasaki YF, Ohsawa K, Yokohama N, Takahashi A, Watanabe T, Mitsumori K | title = Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice | journal = Toxicological Sciences | volume = 69 | issue = 2 | pages = 317–321 | date = October 2002 | pmid = 12377980 | doi = 10.1093/toxsci/69.2.317 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Thomas A, Tocher J, Edwards DI | title = Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli | journal = The Journal of Antimicrobial Chemotherapy | volume = 25 | issue = 5 | pages = 733–744 | date = May 1990 | pmid = 2165050 | doi = 10.1093/jac/25.5.733 }}</ref><ref>{{cite web | url = http://www.academy.org.uk/pharmacy/fluoroq.htm| title = Fluoroquinolones and Quinolones| access-date = 29 January 2009| publisher = The American Academy of Optometry (British Chapter) }}</ref> | ||
| There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.<ref name="pmid14529452"/><ref>{{cite journal |doi=10.1590/S0103-50532003000500014 |title=A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity |year=2003 | |
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.<ref name="pmid14529452"/><ref>{{cite journal |doi=10.1590/S0103-50532003000500014 |title=A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity |year=2003 | vauthors = Al-Soud YA, Al-Masoudi NA |journal=Journal of the Brazilian Chemical Society |volume=14 |issue=5 |pages=790–796|doi-access=free }}</ref> | ||
| ==Adverse reactions== | ==Adverse reactions== | ||
| Flumequine was associated with severe ocular toxicity, which precluded its use in human patients.<ref name=pmid6378414/><ref name=pmid6506018/><ref name=pmid10997595/> Drug-induced calculi (kidney stones) has been associated with such therapy as well.<ref>{{cite journal | |
Flumequine was associated with severe ocular toxicity, which precluded its use in human patients.<ref name=pmid6378414/><ref name=pmid6506018/><ref name=pmid10997595/> Drug-induced calculi (kidney stones) has been associated with such therapy as well.<ref>{{cite journal | vauthors = Daudon M, Protat MF, Réveillaud RJ | title = | language = fr | journal = Annales de Biologie Clinique | volume = 41 | issue = 4 | pages = 239–249 | year = 1983 | pmid = 6139048 }}</ref><ref>{{cite journal | vauthors = Rincé C, Daudon M, Moesch C, Rincé M, Leroux-Robert C | title = Identification of flumequine in a urinary calculus | journal = Journal of Clinical Chemistry and Clinical Biochemistry | volume = 25 | issue = 5 | pages = 313–314 | date = May 1987 | pmid = 3612030 }}</ref><ref>{{cite journal | vauthors = Reveillaud RJ, Daudon M | title = | language = fr | journal = Presse Médicale | volume = 12 | issue = 38 | pages = 2389–2392 | date = October 1983 | pmid = 6138768 }}</ref> Anaphylactic shock induced by flumequine therapy has also been associated with its use.<ref name=pmid1299991/><ref name="Sebbah JL. Presse Med p 28"/><ref>{{cite journal | vauthors = Arboit F, Bessot JC, DeBlay F, Dietemann A, Charpentier C, Pauli G | title = Eight cases of quinolone allergy. | journal = Revue Française d'Allergologie et d'Immunologie Clinique | date = January 1997 | volume = 37 | issue = 1 | pages = 15–19 | doi = 10.1016/S0335-7457(97)80204-3 }}</ref> Anaphylactoid reactions such as shock, urticaria, and Quincke’s oedema have been reported to generally appear within two hours after taking the first tablet. There were eighteen reports listed within the WHO file in 1996.<ref> {{Webarchive|url=https://web.archive.org/web/20120219033146/http://www.who-umc.org/graphics/10575.pdf |date=2012-02-19 }} WHO collaborating centre for international drug monitoring</ref> As with all drugs within this class, flumequine therapy may result in severe central nervous system (CNS) reactions,<ref>{{cite journal | vauthors = Christ W | title = Central nervous system toxicity of quinolones: human and animal findings | journal = The Journal of Antimicrobial Chemotherapy | volume = 26 | issue = Suppl B | pages = 219–225 | date = October 1990 | pmid = 2124211 | doi = 10.1093/jac/26.suppl_b.219 }}</ref><ref>{{cite journal | vauthors = Defoin JF, Debonne T, Rambourg MO, Seraphin J, Buffet M, Jaussaud M, Bertault R, Fay R, Digeon B | display-authors = 6 | title = | language = fr | journal = Journal de Toxicologie Clinique et Expérimentale | volume = 10 | issue = 7–8 | pages = 469–472 | year = 1990 | pmid = 2135062 }}</ref><ref>{{cite journal | vauthors = Rampa S, Caroli F | title = | language = fr | journal = L'Encephale | volume = 17 | issue = 6 | pages = 511–514 | year = 1991 | pmid = 1666873 }}</ref> phototoxicity resulting in skin reactions like erythema, pruritus, urticaria and severe rashes,<ref>{{cite journal | vauthors = Vermeersch G, Filali A, Marko J, Catteau JP, Couture A | title = | language = fr | journal = Journal de Pharmacie de Belgique | volume = 45 | issue = 5 | pages = 299–305 | year = 1990 | pmid = 1964964 }}</ref><ref>{{cite journal | vauthors = Revuz J, Pouget F | title = | language = fr | journal = Annales de Dermatologie et de Venereologie | volume = 110 | issue = 9 | pages = 765 | year = 1983 | pmid = 6660786 }}</ref> gastrointestinal and neurological disorders.<ref name=pmid3356617/> | ||
| Eight cases of quinolone allergy | |||
| F. F. Arboit 1 , JC Bessot 2 , F. Arboit 1, JC Bessot 2, F. De Blay 2 , A. De Blay 2, A. Dietemann 2 , C. Dietemann 2, C. Charpentier 2 and G. Carpenter 2 and G. Pauli 2 , Pauli 2, | |||
| 1 Hôpital Belle-Ile, Service de Pneumologie, 57045 METZ Cedex, France 1 Belle-Isle Hospital, Department of Pneumology, 57045 METZ Cedex, France | |||
| 2 Service de Pneumologie, Pavillon Laennec, Hôpitaux Universitaires de Strasbourg, BP 426, 67091 STRASBOURG Cedex, France 2 Service de Pneumologie, Pavillon Laennec, Hôpitaux Universitaires de Strasbourg, BP 426, 67091 Strasbourg Cedex, France{{Verify source|can't find this title|date=March 2010}}</ref> Anaphylactoid reactions such as shock, urticaria, and Quincke’s oedema have been reported to generally appear within two hours after taking the first tablet. There were eighteen reports listed within the WHO file in 1996.<ref> WHO collaborating centre for international drug monitoring</ref> As with all drugs within this class, flumequine therapy may result in severe central nervous system (CNS) reactions,<ref>{{cite journal |author=Christ W |title=Central nervous system toxicity of quinolones: human and animal findings |journal=The Journal of Antimicrobial Chemotherapy |volume=26 |issue=Suppl B |pages=219–25 |year=1990 |month=October |pmid=2124211 |url=http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=2124211}}</ref><ref>{{cite journal |author=Defoin JF, Debonne T, Rambourg MO, ''et al.'' |title= |language=French |journal=Journal De Toxicologie Clinique et Expérimentale |volume=10 |issue=7-8 |pages=469–72 |year=1990 |pmid=2135062}}</ref><ref>{{cite journal |author=Rampa S, Caroli F |title= |language=French |journal=L'Encéphale |volume=17 |issue=6 |pages=511–4 |year=1991 |pmid=1666873}}</ref> phototoxicity resulting in skin reactions like erythema, pruritus, urticaria and severe rashes,<ref>{{cite journal |author=Vermeersch G, Filali A, Marko J, Catteau JP, Couture A |title= |language=French |journal=Journal De Pharmacie De Belgique |volume=45 |issue=5 |pages=299–305 |year=1990 |pmid=1964964}}</ref><ref>{{cite journal |author=Revuz J, Pouget F |title= |language=French |journal=Annales De Dermatologie et De Vénéréologie |volume=110 |issue=9 |page=765 |year=1983 |pmid=6660786}}</ref> gastrointestinal and neurological disorders.<ref name=pmid3356617/> | |||
| ===History of the black box warnings=== | |||
| {{See also|Quinolone#Black box warnings}} | |||
| Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to ].<ref name="autogenerated604">{{cite journal |author=Bailey RR, Natale R, Linton AL |title=Nalidixic acid arthralgia |journal=Canadian Medical Association Journal |volume=107 |issue=7 |pages=604 passim |year=1972 |month=October |pmid=4541768 |pmc=1940945}}</ref> Rheumatic disease after use of a fluoroquinolone (]) was first reported eleven years later.<ref name="autogenerated590">{{cite journal |author=Bailey RR, Kirk JA, Peddie BA |title=Norfloxacin-induced rheumatic disease |journal=The New Zealand Medical Journal |volume=96 |issue=736 |page=590 |year=1983 |month=July |pmid=6223241}}</ref> In response to a 1995 letter published in the '']'', representatives of the ] (FDA) stated that the agency would "update the labeling for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."<ref name="autogenerated193">{{cite journal |author=Szarfman A, Chen M, Blum MD |title=More on fluoroquinolone antibiotics and tendon rupture |journal=The New England Journal of Medicine |volume=332 |issue=3 |page=193 |year=1995 |month=January |pmid=7800023 |doi=10.1056/NEJM199501193320319}}</ref> | |||
| By August 1996, the FDA had not taken action, and the consumer advocacy group ] filed a petition with the FDA, prompting the agency to act.<ref>{{cite web |title=Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399) |date=August 1, 1996 |publisher=] |url=http://www.citizen.org/publications/release.cfm?ID=6595}} Retrieved on December 27, 2008.</ref> Two months later, the FDA published an alert in the ''FDA Medical Bulletin'' and requested that fluoroquinolone package inserts be amended to include information on this risk.<ref name=FDA1996>{{cite journal |title=Reports of adverse events with fluoroquinolones |journal=FDA Medical Bulletin |date=October 1996 |volume=26 |issue=3 |url=http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc |format=Doc}}</ref> | |||
| Nine years later, in 2005, the ] filed a second petition with the FDA, again seeking ]s and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter.<ref name=Madigan>{{cite press release |title=Madigan, Public Citizen, petition FDA for "Black Box" warning regarding potential adverse effects of certain popular antibiotics |date=August 29, 2006 |publisher=Office of the Illinois Attorney General |url=http://www.illinoisattorneygeneral.gov/pressroom/2006_08/20060829.html | accessdate = 2008-12-27}} Full text of the and available from the Fluoroquinolone Toxicity Research Foundation, a U.S. consumer advocacy group.</ref> In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for Black Box Warnings by filing a third petition requesting such changes be made.<ref name=Madigan/><ref>{{cite web |title=Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781) |date=August 29, 2006 |publisher=Public Citizen |url=http://www.citizen.org/publications/release.cfm?ID=7453 | accessdate = 2008-12-27}}</ref> When the FDA failed to respond to these two petitions as required by law Public Citizen, in January 2008, filed suit to compel the FDA to respond to their 2006 petition.<ref>{{cite web |url=http://www.citizen.org/litigation/forms/cases/CaseDetails.cfm?cID=444 |title=Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone) |date=January 3, 2008 |publisher=Public Citizen | accessdate = 2008-12-27}}</ref><ref>{{cite news |last=Ravn |first=Karen |title=Behind the FDA’s ‘black box’ warnings |date=August 18, 2008 |publisher=] |url=http://articles.latimes.com/2008/aug/18/health/he-closer18 | accessdate = 2008-12-27}}</ref> On July 7, 2008 the FDA requested that the makers of systemic-use fluoroquinolones add a boxed warning regarding spontaneous tendon ruptures, and to develop a Medication Guide for patients.<ref name=FDA>{{cite press release |url=http://www.fda.gov/bbs/topics/NEWS/2008/NEW01858.html |title=FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs |publisher=] |date= 2008-07-08 | accessdate = 2008-10-11}}</ref> The package inserts for Ciprofloxacin, Avelox (]), Proquin XR, Factive (]), Floxin (]), Noroxin (]) and Levaquin (]) were amended on September 8, 2008 to include these new warnings.<ref>The complete labeling history of each drug is available from . Medication Guides are available from the FDA's system.</ref> ], which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes.<ref>{{cite web |last=MacCarthy |first=Paul |title=Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture |date=October 22, 2008 |publisher=Bayer HealthCare Pharmaceuticals |url=http://www.cipro.com/html/pdf/dhpl.pdf | accessdate= 2008-12-27}}</ref> ], the manufacturers of Levaquin, issued a similar letter in November.<ref>{{cite web |last=Rosenthal |first=Norman |title=Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture |date=November 2008 |publisher=Ortho-McNeil Janssen Scientific Affairs, LLC |url=http://www.fqresearch.org/pdf_files/Levaquin_11_2008_ortho_mcneil_dear_dr_letter.pdf | accessdate= 2008-12-27}}</ref> through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals. | |||
| Review of the FDA website indicates that the majority of the generic versions of the fluoroquinolones have ''not'' been updated to include this Black Box Warning as of September 2009. In addition, there are numerous reports that claim that this information has not been dessiminated to the pharmacist, the name brand products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution. | |||
| ==Drug interactions== | ==Drug interactions== | ||
| Flumequine was found to have no effect on theophylline pharmacokinetics.<ref>{{cite journal | |
Flumequine was found to have no effect on ] pharmacokinetics.<ref>{{cite journal | vauthors = Lacarelle B, Blin O, Audebert C, Auquier P, Karsenty H, Horriere F, Durand A | title = The quinolone, flumequine, has no effect on theophylline pharmacokinetics | journal = European Journal of Clinical Pharmacology | volume = 46 | issue = 5 | pages = 477–478 | year = 1994 | pmid = 7957547 | doi = 10.1007/bf00191915 | s2cid = 97621 }}</ref> | ||
| ==Chemistry== | ==Chemistry== | ||
| ⚫ | Flumequine is a 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzoquinolizine-2-carboxylic acid. The molecular formula is C<sub>14</sub>H<sub>12</sub>FNO<sub>3</sub>. It is a white powder, odorless, flavorless, insoluble in water but soluble in organic solvent.<ref>{{cite web|url=http://www.88chem.com/product-Antibiotic-and-Antimicrobial-Agents/10132/Flumequine-antiniotic-antimicrobial-agents-.html |title=Flumequine(antiniotic antimicrobial agents) Manufacturers & Suppliers |publisher=88chem.com |access-date=2010-04-04}}</ref> | ||
| Flumequine is a 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzoquinolizine-2-carboxylic acid. | |||
| ⚫ | |||
| ==Pharmacokinetics == | ==Pharmacokinetics == | ||
| Flumequine is considered to be well absorbed and is excreted in the urine and feces as the glucuronide conjugates of the parent drug and 7-hydroxyflumequine. It is eliminated within 168 hours post-dosing. However, studies concerning the calf liver showed additional unidentified residues, of which a new metabolite, ml, represented the major single metabolite 24 hours after the last dose and at all subsequent time points. The metabolite ml, which exhibited no antimicrobial activity, was present in both free and protein-bound fractions. The major residue found in the edible tissues of sheep, pigs, and chickens was parent drug together with minor amounts of the 7-hydroxy-metabolite. The only detected residue in trout was the parent drug.<ref> |
Flumequine is considered to be well absorbed and is excreted in the urine and feces as the glucuronide conjugates of the parent drug and 7-hydroxyflumequine. It is eliminated within 168 hours post-dosing. However, studies concerning the calf liver showed additional unidentified residues, of which a new metabolite, ml, represented the major single metabolite 24 hours after the last dose and at all subsequent time points. The metabolite ml, which exhibited no antimicrobial activity, was present in both free and protein-bound fractions. The major residue found in the edible tissues of sheep, pigs, and chickens was parent drug together with minor amounts of the 7-hydroxy-metabolite. The only detected residue in trout was the parent drug.<ref>{{cite web | title = Flumequine | vauthors = Francis PG, Wells RJ | publisher = Australian Government Analytical Laboratories | location = Pymble, Australia | url = http://www.fao.org/docrep/W8338E/w8338e0a.htm }}</ref> | ||
| ==See also== | == See also == | ||
| * ] | |||
| *] | |||
| *] | |||
| ==References== | == References == | ||
| {{reflist|colwidth=30em}} | {{reflist|colwidth=30em}} | ||
| {{QuinoloneAntiBiotics}} | {{QuinoloneAntiBiotics}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:26, 4 August 2024
Chemical compound Pharmaceutical compound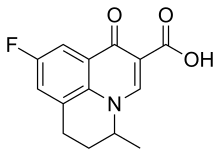 | |
| Clinical data | |
|---|---|
| Other names | 9-Fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzo-quinolizine-2-carboxylic acid |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | urine and feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.857 |
| Chemical and physical data | |
| Formula | C14H12FNO3 |
| Molar mass | 261.252 g·mol |
| 3D model (JSmol) | |
| Melting point | 253 to 255 °C (487 to 491 °F) |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Flumequine is a synthetic fluoroquinolone antibiotic used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. The marketing authorization of flumequine has been suspended throughout the EU. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections (all infections of the intestinal tract), as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France (and a few other European Countries) to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.
History
The first quinolone used was nalidixic acid (was marketed in many countries as Negram) followed by the fluoroquinolone flumequine. The first-generation fluoroquinolone agents, such as flumequine, had poor distribution into the body tissues and limited activity. As such they were used mainly for treatment of urinary tract infections. Flumequine (benzo quinolizine) was first patented in 1973, (German Patent) by Rikker Labs. Flumequine is a known antimicrobial compound described and claimed in U.S. Pat. No. 3,896,131 (Example 3), July 22, 1975. Flumequine is the first quinolone compound with a fluorine atom at the C6-position of the related quinolone basic molecular structure. Even though this was the first fluoroquinolone, it is often overlooked when classifying the drugs within this class by generations and excluded from such a list.
Though used frequently to treat farm animals and on occasion household pets, flumequine was also used to treat urinary tract infections in humans. Flumequine, was used transiently treat urinary infections until ocular toxicity was reported. as well as liver damage and anaphylactic shock.
In 2008, the United States Food and Drug Administration (FDA) requested that all quinolone/fluoroquinolone drugs package inserts include a Black Boxed Warning concerning the risk of spontaneous tendon ruptures, which would have included flumequine. The FDA also requested that the manufacturers send out Dear Doctor Letters regarding this new warning. Such tendon problems have also been associated with flumequine.
Drug residue
The use of flumequine in food animals had sparked considerable debate. Significant and harmful residues of quinolones have been found in animals treated with quinolones and later slaughtered and sold as food products. There has been significant concern regarding the amount of flumequine residue found within food animals such as fish, poultry and cattle. In 2003 the Joint FAO/WHO Committee on Food Additives (JECFA) withdrew the maximum residue limits (MRLs) for flumequine and carbadox based on evidence showing both are direct acting genotoxic carcinogens, therefore the Committee was unable to establish an Acceptable Daily Intake (ADI) for human exposure to such residues. Subsequently, in 2006, the JEFCA, re-established the ADI having received appropriate evidence and MRLs were re-specified. The role of JECFA is to evaluate toxicology, residue chemistry and related information and make recommendations for acceptable daily intake (ADI) levels and maximum residue limits (MRLs). At its 16th session, held May 2006, the Committee on Residues of Veterinary Drugs in Foods (CCRVDF) requested information on registered uses of flumequine. As the CCRVDF did not receive any information regarding the registered uses of flumequine that they had requested, the committee members agreed to discontinue work on the MRLs for flumequine in shrimp.
Licensed uses
Urinary tract infections (veterinary and human)
Availability
Veterinary use:
- Solution; Oral; 20% (prescription only)
- Solution; Oral; 10% (prescription only)
Human use:
- Tablet; Oral; Flumequine 400 mg (discontinued)
Mode of action
Flumequine is a member of the quinolone antibiotics family, which are active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV, enzymes necessary to separate bacterial DNA, thereby inhibiting cell division.
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine), display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models.
Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.).
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.
As such, some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.
Adverse reactions
Flumequine was associated with severe ocular toxicity, which precluded its use in human patients. Drug-induced calculi (kidney stones) has been associated with such therapy as well. Anaphylactic shock induced by flumequine therapy has also been associated with its use. Anaphylactoid reactions such as shock, urticaria, and Quincke’s oedema have been reported to generally appear within two hours after taking the first tablet. There were eighteen reports listed within the WHO file in 1996. As with all drugs within this class, flumequine therapy may result in severe central nervous system (CNS) reactions, phototoxicity resulting in skin reactions like erythema, pruritus, urticaria and severe rashes, gastrointestinal and neurological disorders.
Drug interactions
Flumequine was found to have no effect on theophylline pharmacokinetics.
Chemistry
Flumequine is a 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzoquinolizine-2-carboxylic acid. The molecular formula is C14H12FNO3. It is a white powder, odorless, flavorless, insoluble in water but soluble in organic solvent.
Pharmacokinetics
Flumequine is considered to be well absorbed and is excreted in the urine and feces as the glucuronide conjugates of the parent drug and 7-hydroxyflumequine. It is eliminated within 168 hours post-dosing. However, studies concerning the calf liver showed additional unidentified residues, of which a new metabolite, ml, represented the major single metabolite 24 hours after the last dose and at all subsequent time points. The metabolite ml, which exhibited no antimicrobial activity, was present in both free and protein-bound fractions. The major residue found in the edible tissues of sheep, pigs, and chickens was parent drug together with minor amounts of the 7-hydroxy-metabolite. The only detected residue in trout was the parent drug.
See also
References
- INN: Lomefloxacin Hydrochloride
- Nelson JM, Chiller TM, Powers JH, Angulo FJ (April 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story". Clinical Infectious Diseases. 44 (7): 977–980. doi:10.1086/512369. PMID 17342653.
- Kawahara S (December 1998). "". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 56 (12): 3096–3099. PMID 9883617.
- ^ "Quinolones and fluoroquinolones". Pharmacorama. Archived from the original on 2019-05-12. Retrieved 2010-04-04.
- "Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics". European Medicines Agency. 11 March 2019.
- Francis PG, Wells RJ (1998). "Flumequine". Joint FAO/WHO Expert Committee on Food Additives. Residues of some veterinary drugs in animals and foods. Rome: Food and Agriculture Organization. ISBN 92-5-104128-8. OCLC 39798999.
- Use of quinolones in food animals and potential impact on human health (PDF). WHO meeting. Geneva, Switzerland: World Health Organization. June 1998. Archived from the original (PDF) on 14 January 2010. Cattle only in Europe and Latin America. (Limited use in Latin America), Poultry only in Europe, Asia and Latin America, Fish only in Asia
- Lavenberg DL. "IX4Q 4586, Brrytrit 3.23% Concentrate Solution for Use in Chicken Drinking Water, Genera. Correspondence, Published Literure" (PDF). Bayer. Archived from the original (PDF) on 5 October 2012 – via U.S. Food and Drug Administration.
Quinalones are often used to treat severe cases of human infection with Campylobacter spp., and they are also used in veterinary medicine, especially for treating poultry.
- ^ Schena FP, Gesualdo L, Caracciolo G (January 1988). "A multicentre study of flumequine in the treatment of urinary tract infections". The Journal of Antimicrobial Chemotherapy. 21 (1): 101–106. doi:10.1093/jac/21.1.101. PMID 3356617.
- The quinolones (Third Edition 2000) By Vincent T. Andriole Chapter I History and overview By Dr. Peter Ball (page 5)
- King DE, Malone R, Lilley SH (May 2000). "New classification and update on the quinolone antibiotics". American Family Physician. 61 (9): 2741–2748. PMID 10821154.
- "Generics (UK) Ltd v Daiichi Pharmaceutical Co Ltd". Reports of Patent, Design and Trade Mark Cases. 126 (2): 102. 2009. doi:10.1093/rpc/rcn037.
- "Substituted benzo(ij)quinolizine-2-carboxylic acids and derivatives thereof - Patent 3896131". Freepatentsonline.com. Retrieved 2010-04-04.
- Takahashi H, Hayakawa I, Akimoto T (2003). "". Yakushigaku Zasshi (in Japanese). 38 (2): 161–179. PMID 15143768.
- ^ Sirbat D, Saudax E, Hurault de Ligny B, Hachet E, Abellan P, George JL (1983). "". Bulletin des Sociétés d'Ophtalmologie de France (in French). 83 (8–9): 1019–1021. PMID 6378414.
- ^ Hurault de Ligny B, Sirbat D, Kessler M, Trechot P, Chanliau J (1984). "". Therapie (in French). 39 (5): 595–600. PMID 6506018.
- ^ Ball P (July 2000). "Quinolone generations: natural history or natural selection?". The Journal of Antimicrobial Chemotherapy. 46 (Suppl T1): 17–24. doi:10.1093/oxfordjournals.jac.a020889. PMID 10997595.
- Dubois A, Janbon C, Pignodel C, Marty-Double C (February 1983). "". Gastroenterologie Clinique et Biologique (in French). 7 (2): 217–218. PMID 6840466.
- ^ Pinzani V, Gennaro G, Petit P, Blayac JP (1992). "". Therapie (in French). 47 (5): 440. PMID 1299991.
- ^ Marsepoil T, Blin F, Lo JM, Horel D'Ancona F, Sebbah JL (September 1985). "". Presse Médicale (in French). 14 (32): 1712. PMID 2932732.
- "Fluoroquinolones- A Review– Dr.T R RAMANUJAM.M.D". Medindia.net. Retrieved 2010-04-04.
- Karbiwnyk CM, Hibbard LE, Lee RH, et al. (April 27–28, 2005). Confirmation of Oxolinic Acid and Flumequine Residues in Shrimp by Liquid Chromatography with Tandem Mass Spectrometry Detection. FDA Science.
- "Chemotherapeutics in Seafood Compliance Program: Chapter 4: Pesticides and Chemical Contaminants" (PDF). Compliance Program Guidance Manual. U.S. Food and Drug Administration. 7304.018. Archived from the original (PDF) on 7 March 2011.
Residue found in Catfish/Basa, Shrimp, salmon, trout
- "FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland". Fda.gov. 2009-10-28. Retrieved 2010-04-04.
- "FDA Veterinarian Newsletter, Volume XXII, No. V, 2007" (PDF). Food and Drug Administration. Retrieved 2010-04-04.
- "Codex Committee on Veterinary Drug Residues Acts on Several Documents at 17th Session". Fda.gov. 2009-10-28. Retrieved 2010-04-04.
- WHO Drug Information Vol. 2, No. 3, 1988
- Drlica K, Zhao X (September 1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiology and Molecular Biology Reviews. 61 (3): 377–392. doi:10.1128/mmbr.61.3.377-392.1997. PMC 232616. PMID 9293187.
- Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N (April 1992). "Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group". Antimicrobial Agents and Chemotherapy. 36 (4): 751–756. doi:10.1128/aac.36.4.751. PMC 189387. PMID 1323952.
- ^ Sissi C, Palumbo M (November 2003). "The quinolone family: from antibacterial to anticancer agents". Current Medicinal Chemistry. Anti-Cancer Agents. 3 (6): 439–450. doi:10.2174/1568011033482279. PMID 14529452.
The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models
- Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U (June 1986). "Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts". Antimicrobial Agents and Chemotherapy. 29 (6): 1073–1078. doi:10.1128/AAC.29.6.1073. PMC 180502. PMID 3015015.
- Hosomi J, Maeda A, Oomori Y, Irikura T, Yokota T (1988). "Mutagenicity of Norfloxacin and AM-833 in Bacteria and Mammalian Cells". Reviews of Infectious Diseases. 10 (Supplement 1): S148–9. JSTOR 4454399.
- Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF (May 1987). "Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth". Antimicrobial Agents and Chemotherapy. 31 (5): 774–779. doi:10.1128/AAC.31.5.774. PMC 174831. PMID 3606077.
- Gootz TD, Barrett JF, Sutcliffe JA (January 1990). "Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems". Antimicrobial Agents and Chemotherapy. 34 (1): 8–12. doi:10.1128/AAC.34.1.8. PMC 171510. PMID 2158274.
- Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC (February 1993). "4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells". Journal of Cellular Biochemistry. 51 (2): 165–174. doi:10.1002/jcb.240510208. PMID 8440750. S2CID 41291987.
- Elsea SH, Osheroff N, Nitiss JL (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast". The Journal of Biological Chemistry. 267 (19): 13150–13153. doi:10.1016/S0021-9258(18)42185-0. PMID 1320012.
- Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity". Journal of Medicinal Chemistry. 35 (25): 4745–4750. doi:10.1021/jm00103a013. PMID 1469702.
- Enzmann H, Wiemann C, Ahr HJ, Schlüter G (April 1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver". Mutation Research. 425 (2): 213–224. doi:10.1016/S0027-5107(99)00044-5. PMID 10216214.
- Kashida Y, Sasaki YF, Ohsawa K, Yokohama N, Takahashi A, Watanabe T, Mitsumori K (October 2002). "Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice". Toxicological Sciences. 69 (2): 317–321. doi:10.1093/toxsci/69.2.317. PMID 12377980.
- Thomas A, Tocher J, Edwards DI (May 1990). "Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli". The Journal of Antimicrobial Chemotherapy. 25 (5): 733–744. doi:10.1093/jac/25.5.733. PMID 2165050.
- "Fluoroquinolones and Quinolones". The American Academy of Optometry (British Chapter). Retrieved 29 January 2009.
- Al-Soud YA, Al-Masoudi NA (2003). "A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity". Journal of the Brazilian Chemical Society. 14 (5): 790–796. doi:10.1590/S0103-50532003000500014.
- Daudon M, Protat MF, Réveillaud RJ (1983). "". Annales de Biologie Clinique (in French). 41 (4): 239–249. PMID 6139048.
- Rincé C, Daudon M, Moesch C, Rincé M, Leroux-Robert C (May 1987). "Identification of flumequine in a urinary calculus". Journal of Clinical Chemistry and Clinical Biochemistry. 25 (5): 313–314. PMID 3612030.
- Reveillaud RJ, Daudon M (October 1983). "". Presse Médicale (in French). 12 (38): 2389–2392. PMID 6138768.
- Arboit F, Bessot JC, DeBlay F, Dietemann A, Charpentier C, Pauli G (January 1997). "Eight cases of quinolone allergy". Revue Française d'Allergologie et d'Immunologie Clinique. 37 (1): 15–19. doi:10.1016/S0335-7457(97)80204-3.
- Adverse Reaction Newsletter 1996:1 Archived 2012-02-19 at the Wayback Machine WHO collaborating centre for international drug monitoring
- Christ W (October 1990). "Central nervous system toxicity of quinolones: human and animal findings". The Journal of Antimicrobial Chemotherapy. 26 (Suppl B): 219–225. doi:10.1093/jac/26.suppl_b.219. PMID 2124211.
- Defoin JF, Debonne T, Rambourg MO, Seraphin J, Buffet M, Jaussaud M, et al. (1990). "". Journal de Toxicologie Clinique et Expérimentale (in French). 10 (7–8): 469–472. PMID 2135062.
- Rampa S, Caroli F (1991). "". L'Encephale (in French). 17 (6): 511–514. PMID 1666873.
- Vermeersch G, Filali A, Marko J, Catteau JP, Couture A (1990). "". Journal de Pharmacie de Belgique (in French). 45 (5): 299–305. PMID 1964964.
- Revuz J, Pouget F (1983). "". Annales de Dermatologie et de Venereologie (in French). 110 (9): 765. PMID 6660786.
- Lacarelle B, Blin O, Audebert C, Auquier P, Karsenty H, Horriere F, Durand A (1994). "The quinolone, flumequine, has no effect on theophylline pharmacokinetics". European Journal of Clinical Pharmacology. 46 (5): 477–478. doi:10.1007/bf00191915. PMID 7957547. S2CID 97621.
- "Flumequine(antiniotic antimicrobial agents) Manufacturers & Suppliers". 88chem.com. Retrieved 2010-04-04.
- Francis PG, Wells RJ. "Flumequine". Pymble, Australia: Australian Government Analytical Laboratories.
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||