| Revision as of 19:26, 15 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 08:25, 9 September 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,129 edits removed Category:Chloroarenes; added Category:4-Chlorophenyl compounds using HotCat | ||
| (61 intermediate revisions by 35 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 450690409 | ||
| | IUPAC_name = 1--6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline | | IUPAC_name = 1--6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline | ||
| | image = Methopholine.svg | | image = Methopholine.svg | ||
| | width = 180 | | width = 180 | ||
| Line 14: | Line 15: | ||
| | legal_UK = | | legal_UK = | ||
| | legal_US = | | legal_US = | ||
| | legal_status = |
| legal_status = ? | ||
| | routes_of_administration = |
| routes_of_administration = | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| Line 22: | Line 23: | ||
| | metabolism = | | metabolism = | ||
| | elimination_half-life = | | elimination_half-life = | ||
| | excretion = |
| excretion = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 2154-02-1 | | CAS_number = 2154-02-1 | ||
| | CAS_supplemental = <br /> 63937-57-5 (4'-nitromethopholine) | | CAS_supplemental = <br /> 63937-57-5 (4'-nitromethopholine) | ||
| Line 41: | Line 43: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=20 | H=24 | Cl=1 | N=1 | O=2 | | C=20 | H=24 | Cl=1 | N=1 | O=2 | ||
| | smiles = ClC1=CC=C(CCC2C3=CC(OC)=C(OC)C=C3CCN2C)C=C1 | |||
| | molecular_weight = 345.87 g/mol | |||
| | smiles = Clc1ccc(cc1)CCC3c2c(cc(OC)c(OC)c2)CCN3C | |||
| | InChI = 1/C20H24ClNO2/c1-22-11-10-15-12-19(23-2)20(24-3)13-17(15)18(22)9-6-14-4-7-16(21)8-5-14/h4-5,7-8,12-13,18H,6,9-11H2,1-3H3 | |||
| | InChIKey = YBCPYHQFUMNOJG-UHFFFAOYAD | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C20H24ClNO2/c1-22-11-10-15-12-19(23-2)20(24-3)13-17(15)18(22)9-6-14-4-7-16(21)8-5-14/h4-5,7-8,12-13,18H,6,9-11H2,1-3H3 | | StdInChI = 1S/C20H24ClNO2/c1-22-11-10-15-12-19(23-2)20(24-3)13-17(15)18(22)9-6-14-4-7-16(21)8-5-14/h4-5,7-8,12-13,18H,6,9-11H2,1-3H3 | ||
| Line 52: | Line 51: | ||
| }} | }} | ||
| ''' |
'''Metofoline''' (]), also known as '''methofoline''' (]), is an ] ] drug discovered in the 1950s by a team of Swiss researchers at Hoffmann-La Roche.<ref>{{ cite patent | country = US | status = patent | number = 3067203 | title = Tetrahydroisoquinoline Derivatives | assign1 = Hoffmann-La Roche | gdate = 1962-12-04 }}</ref> | ||
| Methopholine is an ] derivative which is not structurally related to most other opioids.<ref>Feinberg AP, Creese I, Snyder SH |
Methopholine is an ] derivative which is not structurally related to most other opioids.<ref>{{cite journal | vauthors = Feinberg AP, Creese I, Snyder SH | title = The opiate receptor: a model explaining structure-activity relationships of opiate agonists and antagonists | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 73 | issue = 11 | pages = 4215–9 | date = November 1976 | pmid = 186791 | pmc = 431391 | doi = 10.1073/pnas.73.11.4215 | bibcode = 1976PNAS...73.4215F | doi-access = free }}</ref> | ||
| However, its structural similarity to the non-opioid alkaloid ] is notable. | |||
| Metofoline has around the same efficacy as an analgesic as ], and was evaluated for the treatment of postoperative pain.<ref>{{cite journal | vauthors = Moore J, Foldes FF, Davidson GM | title = An evaluation of the efficacy of methopholine for the relief of postoperative pain | journal = The American Journal of the Medical Sciences | volume = 244 | pages = 337–43 | date = September 1962 | issue = 3 | pmid = 14475666 | doi = 10.1097/00000441-196209000-00010 | s2cid = 36562481 }}</ref><ref>{{cite journal | vauthors = Cass LJ, Frederik WS | title = Methopholine, A New Analgesic Agent | journal = The American Journal of the Medical Sciences | volume = 246 | pages = 550–7 | date = November 1963 | issue = 5 | pmid = 14082642 | doi = 10.1097/00000441-196311000-00005 }}</ref><ref>{{cite journal | vauthors = Sciorelli G | title = Plasma levels and renal excretion of unchanged methopholine in man | journal = Experientia | volume = 23 | issue = 11 | pages = 934–6 | date = November 1967 | pmid = 6057015 | doi = 10.1007/bf02136231 | s2cid = 32258639 }}</ref> Metofoline tablets were marketed in the United States under the brand name of Versidyne,<ref>{{cite journal | vauthors = Ryan RE | title = Versidyne. Its use in vascular headache | journal = Headache | volume = 2 | pages = 203–8 | date = January 1963 | issue = 4 | pmid = 13975764 | doi = 10.1111/j.1526-4610.1963.hed0204203.x | s2cid = 2891222 }}</ref> but the drug was withdrawn from the market in 1965 due to the occurrence of ] side-effects alongside the discovery that the drug could produce ] in dogs.<ref>{{Federal Register|30|4083}} March 27, 1965</ref> | |||
| Metofoline has two ]s, with the ''levo'' (R) enantiomer being the active form, around 3x the potency of codeine, and the (S) enantiomer being inactive. | |||
| ⚫ | |||
| ⚫ | ] where the 4'-chloro group has been replaced by other ]s have also been tested, the fluoro derivative being slightly more potent than chloro, and the nitro derivative being most potent of all, with the racemic 4'-nitromethopholine being around 20x the potency of codeine.<ref>{{ cite book | vauthors = Casy AF, Parfitt RY | title = Opioid Analgesics, Chemistry and Receptors | year = 1986 | publisher = Plenum Press | location = New York | page = 390 | isbn = 0-306-42130-5 }}</ref><ref>{{Cite journal | vauthors = Walter M, Besendorf H, Schnider O | doi = 10.1002/hlca.19630460405 | title = Synthesen in der Isochinolinreihe. Substituierte 1--1,2,3,4-tetrahydro-isochinoline | journal = Helvetica Chimica Acta | volume = 46 | issue = 4 | pages = 1127–1132 | year = 1963 }}</ref> | ||
| ⚫ | ] | ||
| Later research was carried out by Bristol-Myer in the 1960s<ref>US Patent 3378561A 1-beta-arylthioethyltetrahydro-isoquinolines</ref> and animal studies suggested derivatives with significantly increased analgesic activity of over x50 codeine.<ref>'Tetrahydroisoquinolines. I. The Preparation and Analgesic Activity of Some 1-Thiophenoxyethyltetrahydroisoquinolines and 1-Phenoxyethyltetrahydroisoquinolines'J. Med. Chem. 1969 Jul;12(4):575-80. doi: 10.1021/jm00304a003.</ref> | |||
| ⚫ | {{ |
||
| ⚫ | ]{{clear-left}} | ||
| == See also == | |||
| * ] | |||
| * ] | |||
| ⚫ | {{clear}} | ||
| == References == | == References == | ||
| {{Reflist|30em}} | |||
| <references /> | |||
| {{Opioidergics}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 08:25, 9 September 2024
Chemical compound Pharmaceutical compound | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H24ClNO2 |
| Molar mass | 345.87 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Metofoline (INN), also known as methofoline (USAN), is an opioid analgesic drug discovered in the 1950s by a team of Swiss researchers at Hoffmann-La Roche.
Methopholine is an isoquinoline derivative which is not structurally related to most other opioids. However, its structural similarity to the non-opioid alkaloid papaverine is notable.
Metofoline has around the same efficacy as an analgesic as codeine, and was evaluated for the treatment of postoperative pain. Metofoline tablets were marketed in the United States under the brand name of Versidyne, but the drug was withdrawn from the market in 1965 due to the occurrence of ophthalmic side-effects alongside the discovery that the drug could produce cataracts in dogs.
Metofoline has two enantiomers, with the levo (R) enantiomer being the active form, around 3x the potency of codeine, and the (S) enantiomer being inactive.
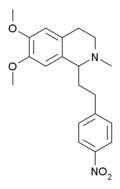
Analogs where the 4'-chloro group has been replaced by other electron withdrawing groups have also been tested, the fluoro derivative being slightly more potent than chloro, and the nitro derivative being most potent of all, with the racemic 4'-nitromethopholine being around 20x the potency of codeine.
Later research was carried out by Bristol-Myer in the 1960s and animal studies suggested derivatives with significantly increased analgesic activity of over x50 codeine.
See also
References
- US patent 3067203, "Tetrahydroisoquinoline Derivatives", issued 1962-12-04, assigned to Hoffmann-La Roche
- Feinberg AP, Creese I, Snyder SH (November 1976). "The opiate receptor: a model explaining structure-activity relationships of opiate agonists and antagonists". Proceedings of the National Academy of Sciences of the United States of America. 73 (11): 4215–9. Bibcode:1976PNAS...73.4215F. doi:10.1073/pnas.73.11.4215. PMC 431391. PMID 186791.
- Moore J, Foldes FF, Davidson GM (September 1962). "An evaluation of the efficacy of methopholine for the relief of postoperative pain". The American Journal of the Medical Sciences. 244 (3): 337–43. doi:10.1097/00000441-196209000-00010. PMID 14475666. S2CID 36562481.
- Cass LJ, Frederik WS (November 1963). "Methopholine, A New Analgesic Agent". The American Journal of the Medical Sciences. 246 (5): 550–7. doi:10.1097/00000441-196311000-00005. PMID 14082642.
- Sciorelli G (November 1967). "Plasma levels and renal excretion of unchanged methopholine in man". Experientia. 23 (11): 934–6. doi:10.1007/bf02136231. PMID 6057015. S2CID 32258639.
- Ryan RE (January 1963). "Versidyne. Its use in vascular headache". Headache. 2 (4): 203–8. doi:10.1111/j.1526-4610.1963.hed0204203.x. PMID 13975764. S2CID 2891222.
- 30 FR 4083 March 27, 1965
- Casy AF, Parfitt RY (1986). Opioid Analgesics, Chemistry and Receptors. New York: Plenum Press. p. 390. ISBN 0-306-42130-5.
- Walter M, Besendorf H, Schnider O (1963). "Synthesen in der Isochinolinreihe. Substituierte 1--1,2,3,4-tetrahydro-isochinoline". Helvetica Chimica Acta. 46 (4): 1127–1132. doi:10.1002/hlca.19630460405.
- US Patent 3378561A 1-beta-arylthioethyltetrahydro-isoquinolines
- 'Tetrahydroisoquinolines. I. The Preparation and Analgesic Activity of Some 1-Thiophenoxyethyltetrahydroisoquinolines and 1-Phenoxyethyltetrahydroisoquinolines'J. Med. Chem. 1969 Jul;12(4):575-80. doi: 10.1021/jm00304a003.