| Revision as of 20:48, 20 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProj← Previous edit |
Latest revision as of 17:08, 21 January 2025 edit undoFswitzer4 (talk | contribs)Extended confirmed users10,990 editsm Added UNII ID |
| (34 intermediate revisions by 20 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Chemical compound}} |
|
{{Drugbox |
|
{{Drugbox |
|
|
| Verifiedfields = changed |
| ⚫ |
| verifiedrevid = 447295874 |
|
|
|
| Watchedfields = changed |
|
⚫ |
| verifiedrevid = 451564438 |
|
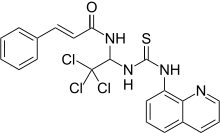
| IUPAC_name = 3-phenyl-''N''-[2,2,2-trichloro-1-[[(8-quinolinylamino)thioxomethyl]amino]ethyl]-2-propenamide |
|
| IUPAC_name = 3-phenyl-''N''-[2,2,2-trichloro-1-[[(8-quinolinylamino)thioxomethyl]amino]ethyl]-2-propenamide |
|
| image = Salubrinal.svg |
|
| image = Salubrinal.svg |
|
| width = 200 |
|
| width = |
|
| alt = |
|
| alt = |
|
| image2 = |
|
| image2 = |
|
| width2 = |
|
| width2 = |
|
|
| drug_name = |
|
| imagename = <!-- else may use drug_name --> |
|
|
| drug_name = <!-- else may use imagename --> |
|
|
|
|
|
|
<!--Clinical data--> |
|
<!--Clinical data--> |
| Line 24: |
Line 26: |
|
| legal_status = |
|
| legal_status = |
|
| dependency_liability = |
|
| dependency_liability = |
|
| routes_of_administration = |
|
| routes_of_administration = |
|
|
|
|
<!--Pharmacokinetic data--> |
|
<!--Pharmacokinetic data--> |
|
| bioavailability = |
|
| bioavailability = |
| Line 31: |
Line 32: |
|
| metabolism = |
|
| metabolism = |
|
| elimination_half-life = |
|
| elimination_half-life = |
|
| excretion = |
|
| excretion = |
|
|
|
|
<!--Identifiers--> |
|
<!--Identifiers--> |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number = 405060-95-9 |
|
| CAS_number = 405060-95-9 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = J8PSF5Z8KJ |
|
| CAS_supplemental = |
|
| CAS_supplemental = |
|
| ATCvet = |
|
| ATCvet = |
| Line 40: |
Line 43: |
|
| ATC_suffix = |
|
| ATC_suffix = |
|
| ATC_supplemental = |
|
| ATC_supplemental = |
|
| PubChem = |
|
| PubChem = 5717801 |
|
⚫ |
| DrugBank = |
|
| PubChemSubstance = |
|
|
⚫ |
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|
| IUPHAR_ligand = |
|
|
⚫ |
| ChemSpiderID = 4654442 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
|
|
| ChEBI_Ref = {{ebicite|changed|EBI}} |
| ⚫ |
| DrugBank = |
|
|
|
| ChEBI = 131923 |
| ⚫ |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
|
|
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
| ⚫ |
| ChemSpiderID = |
|
|
| synonyms = |
|
| ChEMBL = 180127 |
|
|
| synonyms = |
|
|
|
|
<!--Chemical data--> |
|
<!--Chemical data--> |
|
| chemical_formula = |
|
| chemical_formula = |
|
| C=21 | H=17 | Cl=3 | N=4 | O=1 | S=1 |
|
| C=21 | H=17 | Cl=3 | N=4 | O=1 | S=1 |
|
|
| SMILES = C1=CC=C(C=C1)/C=C/C(=O)NC(C(Cl)(Cl)Cl)NC(=S)NC2=CC=CC3=C2N=CC=C3 |
|
| molecular_weight = 479.81 <ref name="cdata1"/><ref name="cdata2"/> |
|
|
|
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|
| smiles = |
|
|
|
| StdInChI = 1S/C21H17Cl3N4OS/c22-21(23,24)19(27-17(29)12-11-14-6-2-1-3-7-14)28-20(30)26-16-10-4-8-15-9-5-13-25-18(15)16/h1-13,19H,(H,27,29)(H2,26,28,30)/b12-11+ |
|
|
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|
|
| StdInChIKey = LCOIAYJMPKXARU-VAWYXSNFSA-N |
|
| density = |
|
| density = |
|
| melting_point = |
|
| melting_point = |
| Line 65: |
Line 71: |
|
}} |
|
}} |
|
|
|
|
|
'''Salubrinal''' is a drug which acts as a specific inhibitor of ] ] enzymes<ref name="jl1"/><ref name="dk1"/><ref name="bm1"/> and is primarily used experimentally, to study stress responses in ] ]s associated with the action of eIF2. Salbrinal indirectly inhibits eIF2 as a result of reduced ] of its α-],<ref name="entrez1"/> resulting in activation of stress response pathways usually triggered by events such as ] or buildup of unfolded ] in the ].<ref name="rw1"/> Salubrinal has putative ] value due to its function,<ref name="jl1"/><ref name="bm1"/> but is as yet only used experimentally. Salubrinal is being studied at Indiana University for its potential to fight osteoporosis and accelerate bone healing.<ref name="seet1"/> |
|
'''Salubrinal''' is a drug which acts as a specific inhibitor of ] ] enzymes<ref name="jl1"/><ref name="dk1"/><ref name="bm1"/> and is primarily used experimentally, to study stress responses in ] ]s associated with the action of eIF2. Salubrinal indirectly inhibits eIF2 as a result of reduced ] of its α-],<ref name="entrez1"/> resulting in activation of stress response pathways usually triggered by events such as ] or buildup of unfolded ] in the ].<ref name="rw1"/> Salubrinal has putative ] value due to its function,<ref name="jl1"/><ref name="bm1"/> but is as yet only used experimentally. Salubrinal is being studied at Indiana University for its potential to fight osteoporosis and accelerate bone healing.<ref name="seet1"/> |
|
|
|
|
⚫ |
== References == |
|
|
{{Portal|Medicine}} |
|
⚫ |
{{Reflist|refs= |
|
|
|
|
|
<ref name="dk1">{{cite journal | vauthors = Kessel D | title = Protection of Bcl-2 by salubrinal | journal = Biochemical and Biophysical Research Communications | volume = 346 | issue = 4 | pages = 1320–3 | date = August 2006 | pmid = 16806073 | pmc = 2978664 | doi = 10.1016/j.bbrc.2006.06.056 | publisher = Elsevier Inc }}{{dead link|date=March 2019|bot=medic}}{{cbignore|bot=medic}}</ref> |
|
|
|
|
⚫ |
<ref name="jl1">{{cite journal | vauthors = Lewerenz J, Maher P | title = Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression | journal = The Journal of Biological Chemistry | volume = 284 | issue = 2 | pages = 1106–15 | date = January 2009 | pmid = 19017641 | pmc = 2613630 | doi = 10.1074/jbc.M807325200 | publisher = The American Society for Biochemistry and Molecular Biology, Inc | doi-access = free }}</ref> |
|
|
|
|
⚫ |
<ref name="bm1">{{cite journal | vauthors = Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J | display-authors = 6 | title = A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress | journal = Science | volume = 307 | issue = 5711 | pages = 935–9 | date = February 2005 | pmid = 15705855 | doi = 10.1126/science.1101902 | bibcode = 2005Sci...307..935B | s2cid = 86257684 | url = http://www.sciencemag.org/cgi/content/abstract/307/5711/935 }}</ref> |
|
|
|
|
⚫ |
<ref name="entrez1">{{cite web|title=Entrez Gene: EIF2S1 eukaryotic translation initiation factor 2, subunit 1 alpha, 35kDa|url=https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1965|access-date=2010-10-05|publisher= ], U.S. National Library of Medicine}}</ref> |
|
|
|
|
⚫ |
<!-- <ref name="cdata1">{{cite web|title=SALUBRINAL | 405060-95-9|url=http://www.chemicalbook.com/ChemicalProductProperty_EN_CB3322523.htm|access-date=2010-10-05|publisher=ChemicalBook}}</ref> --> |
|
|
|
|
⚫ |
<!-- <ref name="cdata2">{{cite web|url=http://datasheets.scbt.com/sc-202332.pdf|title=Salubrinal: sc-202332|date=2010-07-28|access-date=2010-10-05|publisher=Santa Cruz Biotechnology, Inc}}</ref> --> |
|
|
|
|
|
<ref name="rw1">{{cite journal | vauthors = Wek RC, Jiang HY, Anthony TG | title = Coping with stress: eIF2 kinases and translational control | journal = Biochemical Society Transactions | volume = 34 | issue = Pt 1 | pages = 7–11 | date = February 2006 | pmid = 16246168 | doi = 10.1042/BST20060007 | publisher = Biochemical Society }}</ref> |
|
|
|
|
⚫ |
<ref name="seet1">{{cite web | title=New compound may accelerate bone healing, prevent osteoporosis| url = http://www.cloningresources.com/research/New_compound_may_accelerate_bone_healing_prevent_osteoporosis.asp | archive-url = https://web.archive.org/web/20110929162126/http://www.cloningresources.com/research/New_compound_may_accelerate_bone_healing_prevent_osteoporosis.asp | archive-date = 29 September 2011 |access-date=2011-08-29 |date=August 2011}}</ref> |
|
|
|
|
| ⚫ |
==References== |
|
| ⚫ |
{{Reflist|2|refs= |
|
|
<ref name="dk1">{{cite journal|url=http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WBK-4K714XJ-5&_user=10&_coverDate=08%2F11%2F2006&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1485420146&_rerunOrigin=google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=6afae3548902ab4fd881629a079b171b&searchtype=a|accessdate=2010-10-05|first=David|last=Kessel|publisher=Elsevier Inc|date=2006-08-11|page=1320|volume=346|issue=4|title=Protection of Bcl-2 by sulubrinal|journal=Biochemical and Biophysical Research Communications|doi=10.1016/j.bbrc.2006.06.056|pmid=16806073}}</ref> |
|
| ⚫ |
<ref name="jl1">{{cite journal|title=Basal Levels of eIF2α Phosphorylation Determine Cellular Antioxidant Status by Regulating ATF4 and xCT Expression|journal=]|volume=284|issue=2|page=1106|date=2009-01-09|publisher=The American Society for Biochemistry and Molecular Biology, Inc|first1=Jan|last1=Lewerenz|first2=Pamela|last2=Maher|doi=10.1074/jbc.M807325200}}</ref> |
|
| ⚫ |
<ref name="bm1">{{cite journal|author=Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J|title=A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress|journal=]|volume=307|issue=5711|pages=935–9|year=2005|month=February|pmid=15705855|doi=10.1126/science.1101902|url=http://www.sciencemag.org/cgi/content/abstract/307/5711/935|accessdate=2010-10-05}}</ref> |
|
| ⚫ |
<ref name="entrez1">{{cite web|title=Entrez Gene: EIF2S1 eukaryotic translation initiation factor 2, subunit 1 alpha, 35kDa|url=http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1965|accessdate=2010-10-05|publisher= ], U.S. National Library of Medicine}}</ref> |
|
| ⚫ |
<ref name="cdata1">{{cite web|title=SALUBRINAL | 405060-95-9|url=http://www.chemicalbook.com/ChemicalProductProperty_EN_CB3322523.htm|accessdate=2010-10-05|publisher=ChemicalBook}}</ref> |
|
| ⚫ |
<ref name="cdata2">{{cite web|url=http://datasheets.scbt.com/sc-202332.pdf|title=Salubrinal: sc-202332|date=2010-07-28|accessdate=2010-10-05|publisher=Santa Cruz Biotechnology, Inc}}</ref> |
|
|
<ref name="rw1">{{cite journal|title=Coping with stress: eIF2 kinases and translational control|url=http://www.biochemsoctrans.org/bst/034/0007/0340007.pdf|accessdate=2010-10-05|year=2006|month=February|authors=Wek R, Jiang H, Anthony T|journal=]|pages=7–11|pmid=16246168|volume=34|issue=1|publisher=Biochemical Society}}</ref> |
|
| ⚫ |
<ref name="seet1">{{cite web | title=New compound may accelerate bone healing, prevent osteoporosis| url = http://www.cloningresources.com/research/New_compound_may_accelerate_bone_healing_prevent_osteoporosis.asp |accessdate=2011-29-08 |year=2011 |month=august}}</ref> |
|
|
}} |
|
}} |
|
|
|
|
| Line 83: |
Line 99: |
|
] |
|
] |
|
] |
|
] |
|
|
|
|
|
|
|
{{pharma-stub}} |
|