| Revision as of 02:15, 21 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to watched fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors o← Previous edit |
Latest revision as of 11:16, 25 May 2023 edit undoMykhal (talk | contribs)Extended confirmed users5,586 edits added Category:Imidazothiazoles using HotCat |
| (18 intermediate revisions by 13 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Organic compound, experimental pharmaceuticum}} |
|
{{Drugbox |
|
{{Drugbox |
|
⚫ |
| verifiedrevid = 477856488 |
|
| Watchedfields = changed |
|
|
⚫ |
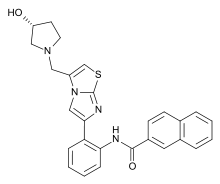
| IUPAC_name = N-methyl}imidazothiazol-6-yl)phenyl]naphthalene-2-carboxamide |
| ⚫ |
| verifiedrevid = 451167855 |
|
| ⚫ |
| IUPAC_name = N-methyl}imidazothiazol-6-yl)phenyl]naphthalene-2-carboxamide |
|
|
| image = SRT2183 skeletal.svg |
|
| image = SRT2183 skeletal.svg |
|
|
|
|
| Line 15: |
Line 15: |
|
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| legal_status = Investigational |
|
| legal_status = Investigational |
|
| routes_of_administration = |
|
| routes_of_administration = |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
<!--Pharmacokinetic data--> |
| Line 22: |
Line 22: |
|
| metabolism = |
|
| metabolism = |
|
| elimination_half-life = |
|
| elimination_half-life = |
|
| excretion = |
|
| excretion = |
|
|
|
|
|
<!--Identifiers--> |
|
<!--Identifiers--> |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number = |
|
| CAS_number = 1001908-89-9 |
|
| ATC_prefix = None |
|
| ATC_prefix = None |
|
| ATC_suffix = |
|
| ATC_suffix = |
|
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEMBL = 403308 |
|
| PubChem = 24180126 |
|
| PubChem = 24180126 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 6FKU9G9CX6 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank = |
|
| DrugBank = |
| Line 36: |
Line 41: |
|
<!--Chemical data--> |
|
<!--Chemical data--> |
|
| C=27 | H=24 | N=4 | O=2 | S=1 |
|
| C=27 | H=24 | N=4 | O=2 | S=1 |
|
| molecular_weight = 468.570 g/mol |
|
|
| smiles = c1ccc2cc(ccc2c1)C(=O)Nc3ccccc3c4cn5c(csc5n4)CN6CC(C6)O |
|
| smiles = c1ccc2cc(ccc2c1)C(=O)Nc3ccccc3c4cn5c(csc5n4)CN6CC(C6)O |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| Line 44: |
Line 48: |
|
}} |
|
}} |
|
|
|
|
|
'''SRT2183''' is a drug in development by ] intended as a ] ] of the ] subtype ]. It has similar activity in the body to another SIRT1 activator ], but is closer in potency to ]. In animal studies it was found to improve ] and lower ] levels in fat, muscle and liver tissue, and increased ] and ].<ref name="pmid18046409">{{cite journal | author = Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH | title = Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes | journal = Nature | volume = 450 | issue = 7170 | pages = 712–6 | year = 2007 | month = November | pmid = 18046409 | pmc = 2753457 | doi = 10.1038/nature06261 | url = | issn = }}</ref> |
|
'''SRT-2183''' is a drug in development by ] intended as a ] ] of the ] subtype ]. It has similar activity in animal studies to another SIRT1 activator ], but is closer in potency to ]. In animal studies it was found to improve ] and lower ] levels in fat, muscle and liver tissue, and increased ] and ].<ref name="pmid18046409">{{cite journal | vauthors = Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH | display-authors = 6 | title = Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes | journal = Nature | volume = 450 | issue = 7170 | pages = 712–6 | date = November 2007 | pmid = 18046409 | pmc = 2753457 | doi = 10.1038/nature06261 | bibcode = 2007Natur.450..712M }}</ref> |
|
However, the claim that SRT2183 is a SIRT1 activator has been questioned<ref name="pmid20061378">{{cite journal | author = Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K | title = SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1 | journal = J Biol Chem | volume = 285| issue = 11| pages = 8340–8351| year = 2010 | month = January | pmid = 20061378 | doi = 10.1074/jbc.M109.088682 | url = | issn = | pmc=2832984}}</ref> and further defended.<ref name="pmid20702418">{{cite journal | author = Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL | title = SIRT1 activation by small molecules - kinetic and biophysical evidence for direct interaction of enzyme and activator | journal = J Biol Chem | volume = 285| issue = 43| pages = 32695–32703| year = 2010 | month = August | pmid = 20702418 | doi = 10.1074/jbc.M110.133892 | url = | issn = | pmc = 2963390 }}</ref> |
|
However, the claim that SRT-2183 is a SIRT1 activator has been questioned<ref name="pmid20061378">{{cite journal | vauthors = Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K | display-authors = 6 | title = SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1 | journal = The Journal of Biological Chemistry | volume = 285 | issue = 11 | pages = 8340–51 | date = March 2010 | pmid = 20061378 | pmc = 2832984 | doi = 10.1074/jbc.M109.088682 | doi-access = free }}</ref> and further defended.<ref name="pmid20702418">{{cite journal | vauthors = Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL | display-authors = 6 | title = SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator | journal = The Journal of Biological Chemistry | volume = 285 | issue = 43 | pages = 32695–703 | date = October 2010 | pmid = 20702418 | pmc = 2963390 | doi = 10.1074/jbc.M110.133892 | doi-access = free }}</ref> |
|
|
|
|
|
==See also== |
|
== See also == |
|
*] |
|
*] |
|
|
|
|
|
==References== |
|
== References == |
|
{{Reflist}} |
|
{{Reflist}} |
|
|
|
|
|
] |
|
] |
|
|
] |
|
|
|
|
|
|
|
|
{{gastrointestinal-drug-stub}} |
|
{{gastrointestinal-drug-stub}} |