| Revision as of 14:44, 4 October 2011 editLuckas-bot (talk | contribs)929,662 editsm r2.7.1) (Robot: Adding vi:Natri xiclopentađienua← Previous edit | Latest revision as of 23:25, 16 December 2024 edit undoLeo51db (talk | contribs)2 edits Image of anion replaced with picture of real life product as the structure is already well represented in the other pictures. | ||
| (72 intermediate revisions by 42 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Redirect|NaCp|other uses|NACP (disambiguation)}} | |||
| {{merge|Cyclopentadienyl radical|Cyclopentadienyl anion|discuss=Talk: Cyclopentadienyl anion#Merge proposal|date=November 2024}} | |||
| {{chembox | {{chembox | ||
| |Watchedfields = changed | |||
| | |
|verifiedrevid = 418117276 | ||
| ⚫ | | |
||
| |ImageFile = Sodium cyclopentadiene.svg | |||
| |ImageSize= | |ImageSize = 100px | ||
| | |
|ImageFile1 = NaCp-chain-from-xtal-3D-balls-C.png | ||
| |ImageSize2=250px | |||
| |ImageFileL2 = NaCp-xtal-3D-SF-A.png | |||
| |IUPACName= | |||
| |ImageFileR2 = NaCp-xtal-3D-SF-B.png | |||
| ⚫ | |OtherNames= |
||
| |ImageFile3 = NaCp Product2.jpg | |||
| |ImageCaption3 = NaCp synthesized in an inert atmosphere | |||
| ⚫ | |PIN = Sodium cyclopentadienide | ||
| ⚫ | |OtherNames = Sodium cyclopentadienylide, Cyclopentadienylsodium | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| ⚫ | |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|StdInChI = 1S/C5H5.Na/c1-2-4-5-3-1;/h1-5H;/q-1;+1 | ||
| ⚫ | | |
||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| ⚫ | | |
||
| | |
|StdInChIKey = OHUVHDUNQKJDKW-UHFFFAOYSA-N | ||
| ⚫ | |InChI = 1S/C5H5.Na/c1-2-4-5-3-1;/h1-5H;/q-1;+1 | ||
| ⚫ | | |
||
| ⚫ | |InChIKey1 = OHUVHDUNQKJDKW-UHFFFAOYSA-N | ||
| ⚫ | | |
||
| ⚫ | |CASNo = 4984-82-1 | ||
| | PubChem=78681 | |||
| ⚫ | |CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | |
||
| |PubChem = 78681 | |||
| ⚫ | | |
||
| ⚫ | |EINECS = 225-636-8 | ||
| ⚫ | | |
||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ⚫ | | |
||
| ⚫ | |ChemSpiderID = 71032 | ||
| ⚫ | |SMILES = .c1ccc1 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
|C=5 | H=5 | Na=1 | ||
| | |
|Appearance=colorless solid | ||
| | |
|Density=1.113 g/cm<sup>3</sup> | ||
| ⚫ | |Solubility=decomposition | ||
| | MeltingPt= | |||
| ⚫ | |SolubleOther=] | ||
| | BoilingPt= | |||
| ⚫ | }} | ||
| ⚫ | | |
||
| | SolubleOther=Soluble | |||
| ⚫ | | |
||
| ⚫ | |||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| | |
|MainHazards=flammable | ||
| ⚫ | }} | ||
| | FlashPt= | |||
| | Autoignition= | |||
| ⚫ | |||
| }} | }} | ||
| '''Sodium cyclopentadienide''' is an ] with the ] C<sub>5</sub>H<sub>5</sub>Na. |
'''Sodium cyclopentadienide''' is an ] with the ] C<sub>5</sub>H<sub>5</sub>Na. The compound is often abbreviated as NaCp, where Cp<sup>−</sup> is the cyclopentadienide anion.<ref>{{RedBookRef2005|page=262}}</ref> Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.<ref name= Roesky/> | ||
| ==Preparation== | ==Preparation== | ||
| Sodium cyclopentadienide is commercially available as a solution in ]. It is prepared by treating ] with ]:<ref>{{Cotton&Wilkinson5th|page=139}}</ref> | Sodium cyclopentadienide is commercially available as a solution in ]. It is prepared by treating ] with ]:<ref>{{Cotton&Wilkinson5th|page=139}}</ref> | ||
| :{{chem2|2 Na + 2 C5H6 -> 2 NaC5H5 + H2}} | |||
| :2 Na + 2 C<sub>5</sub>H<sub>6</sub> → 2 NaC<sub>5</sub>H<sub>5</sub> + ] | |||
| The conversion can be conducted by heating a suspension of molten sodium in ].<ref name= Roesky>Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878.{{doi|10.1021/om0207865}}</ref> In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing ] and rapidly stirring.<ref name = ferrocene>{{OrgSynth | title = Ferrocene | author = ] | collvol = 4 | collvolpages = 473 | year = 1963 | prep = cv4p0473}}</ref><ref>{{OrgSynth | author = Partridge, John J.; Chadha, Naresh K.; Uskokovic, Milan R. | title = An asymmetric hydroboration of 5-substituted cyclopentadienes: synthesis of methyl (1''R'',5''R'')-5-hydroxy-2-cyclopentene-1-acetate | collvol = 7 | collvolpages = 339 | prep = cv7p0339 | year = 1990}}</ref> ] is a convenient base:<ref>{{cite book |author1=Girolami, G. S. |author2=Rauchfuss, T. B. |author3=Angelici, R. J. |author1-link=Gregory S. Girolami |author3-link=Robert Angelici |name-list-style=amp | title = Synthesis and Technique in Inorganic Chemistry | publisher = University Science Books: Mill Valley | location = CA | year = 1999 | isbn = 0935702482}}</ref> | |||
| :{{chem2|NaH + 2 C5H6 -> NaC5H5 + H2}} | |||
| :NaH + C<sub>5</sub>H<sub>6</sub> → NaC<sub>5</sub>H<sub>5</sub> + H<sub>2</sub> | |||
| In early work, ]s were used as bases. |
In early work, ]s were used as bases. With a ] of 15, cyclopentadiene can be deprotonated by many reagents. | ||
| ⚫ | The nature of NaCp depends strongly its medium and for the purposes of planning syntheses |
||
| ==Applications== | ==Applications== | ||
| Sodium cyclopentadienide is a common reagent for the preparation of ]s. For example, the preparation of ]<ref name = ferrocene/> and ]:<ref>{{cite journal | |
Sodium cyclopentadienide is a common reagent for the preparation of ]s. For example, the preparation of ]<ref name = ferrocene/> and ]:<ref>{{cite journal |author1=Wilkinson, G. |author2=Birmingham, J. G. | year = 1954 | title = Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta | journal = ] | volume = 76 | issue = 17 | pages = 4281–84 | doi = 10.1021/ja01646a008|bibcode=1954JAChS..76.4281W |author1-link=Geoffrey Wilkinson }}</ref> | ||
| :{{Chem2|2 NaC5H5 + FeCl2 -> Fe(C5H5)2 + 2 NaCl}} | |||
| :2 NaC<sub>5</sub>H<sub>5</sub> + ] → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 2 NaCl | |||
| : |
:{{chem2|ZrCl4(thf)2 + 2 NaCp -> (C5H5)2ZrCl2 + 2 NaCl + 2 THF}} | ||
| Sodium cyclopentadienide is also used for the preparation of substituted cyclopentadienyl derivatives such as the ester and formyl derivatives:<ref>{{cite journal|author1=Macomber, D. W. |author2=Hart, W. P. |author3=Rausch, M. D. |title=Functionally Substituted Cyclopentadienyl Metal Compounds|journal=Adv. Organomet. Chem.|year=1982|volume=21|pages=1–55|doi=10.1016/S0065-3055(08)60377-9|series=Advances in Organometallic Chemistry |isbn=9780120311217}}</ref> | |||
| :{{chem2|NaC5H5 + O\dC(OEt)2 → NaC5H4CO2Et + NaOEt}} | |||
| These compounds are used to prepare substituted metallocenes such as ].<ref>{{cite journal |doi=10.1021/om4004972|title=Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives |year=2013 |last1=Petrov |first1=Alex R. |last2=Jess |first2=Kristof |last3=Freytag |first3=Matthias |last4=Jones |first4=Peter G. |last5=Tamm |first5=Matthias |journal=Organometallics |volume=32 |issue=20 |pages=5946–5954}}</ref> | |||
| ==Structure== | |||
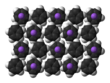
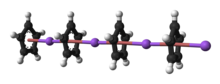
| ⚫ | The nature of NaCp depends strongly on its medium and for the purposes of planning syntheses; the reagent is often represented as a salt {{chem|Na|+|C|5|H|5|-}}. Crystalline solvent-free NaCp, which is rarely encountered, is a "polydecker" ], consisting of an infinite chain of alternating Na<sup>+</sup> centers sandwiched between ]-]:''η''<sup>5</sup>-C<sub>5</sub>H<sub>5</sub> ligands.<ref>{{cite journal |author1=Robert E. Dinnebier |author2=Ulrich Behrens |author3=Falk Olbrich |name-list-style=amp | title = Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. Determination by High-Resolution Powder Diffraction | journal = ] | year = 1997 | volume = 16 |issue=17 | pages = 3855–3858 | doi = 10.1021/om9700122}}</ref> As a solution in donor solvents, NaCp is highly solvated, especially at the alkali metal as suggested by the isolability of the ] Na(])Cp.<ref>{{cite book | author = Elschenbroich, C. | title = Organometallics | year = 2006 | publisher = Wiley-VCH: Weinheim | isbn = 978-3-527-29390-2}}</ref> | ||
| In contrast to alkali metal cyclopentadienides, ] cyclopentadienide (Bu<sub>4</sub>N<sup>+</sup>C<sub>5</sub>H<sub>5</sub><sup>−</sup>) was found to be supported entirely by ionic bonding and its structure is representative of the structure of the cyclopentadienide anion (C<sub>5</sub>H<sub>5</sub><sup>−</sup>, Cp<sup>−</sup>) in the solid state. However, the anion deviates somewhat from a planar, regular pentagon, with C–C bond lengths ranging from 138.0 -140.1 pm and C–C–C bond angles ranging from 107.5-108.8°.<ref>{{cite journal|last1=Reetz|first1=Manfred T.|last2=Hütte|first2=Stephan|last3=Goddard|first3=Richard|date=1995-03-01|title=Tetrabutylammonium Salts of 2-Nitropropane, Cyclopentadiene and 9-Ethylfluorene: Crystal Structures and Use in Anionic Polymerization|journal=Zeitschrift für Naturforschung B|language=en|volume=50|issue=3|pages=415–422|doi=10.1515/znb-1995-0316|s2cid=45791403 |issn=1865-7117|doi-access=free}}</ref> | |||
| ==See also== | |||
| *] | |||
| *] | |||
| ==References== | ==References== | ||
| {{reflist}} | |||
| <references/> | |||
| {{Cyclopentadiene complexes}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 23:25, 16 December 2024
"NaCp" redirects here. For other uses, see NACP (disambiguation).| It has been suggested that this article be merged with Cyclopentadienyl radical and Cyclopentadienyl anion. (Discuss) Proposed since November 2024. |

| |||
| |||
| |||
 NaCp synthesized in an inert atmosphere | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Sodium cyclopentadienide | |||
| Other names Sodium cyclopentadienylide, Cyclopentadienylsodium | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.023.306 | ||
| EC Number |
| ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H5Na | ||
| Molar mass | 88.085 g·mol | ||
| Appearance | colorless solid | ||
| Density | 1.113 g/cm | ||
| Solubility in water | decomposition | ||
| Solubility | THF | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | flammable | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
Preparation
Sodium cyclopentadienide is commercially available as a solution in THF. It is prepared by treating cyclopentadiene with sodium:
- 2 Na + 2 C5H6 → 2 NaC5H5 + H2
The conversion can be conducted by heating a suspension of molten sodium in dicyclopentadiene. In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring. Sodium hydride is a convenient base:
- NaH + 2 C5H6 → NaC5H5 + H2
In early work, Grignard reagents were used as bases. With a pKa of 15, cyclopentadiene can be deprotonated by many reagents.
Applications
Sodium cyclopentadienide is a common reagent for the preparation of metallocenes. For example, the preparation of ferrocene and zirconocene dichloride:
- 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
- ZrCl4(thf)2 + 2 NaCp → (C5H5)2ZrCl2 + 2 NaCl + 2 THF
Sodium cyclopentadienide is also used for the preparation of substituted cyclopentadienyl derivatives such as the ester and formyl derivatives:
- NaC5H5 + O=C(OEt)2 → NaC5H4CO2Et + NaOEt
These compounds are used to prepare substituted metallocenes such as 1,1'-ferrocenedicarboxylic acid.
Structure
The nature of NaCp depends strongly on its medium and for the purposes of planning syntheses; the reagent is often represented as a salt Na
C
5H
5. Crystalline solvent-free NaCp, which is rarely encountered, is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Na centers sandwiched between μ-η:η-C5H5 ligands. As a solution in donor solvents, NaCp is highly solvated, especially at the alkali metal as suggested by the isolability of the adduct Na(tmeda)Cp.
In contrast to alkali metal cyclopentadienides, tetrabutylammonium cyclopentadienide (Bu4NC5H5) was found to be supported entirely by ionic bonding and its structure is representative of the structure of the cyclopentadienide anion (C5H5, Cp) in the solid state. However, the anion deviates somewhat from a planar, regular pentagon, with C–C bond lengths ranging from 138.0 -140.1 pm and C–C–C bond angles ranging from 107.5-108.8°.
See also
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 262. Electronic version.
- ^ Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878.doi:10.1021/om0207865
- Cotton, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (5th ed.), New York: Wiley-Interscience, p. 139, ISBN 0-471-84997-9
- ^ Wilkinson, Geoffrey (1963). "Ferrocene". Organic Syntheses; Collected Volumes, vol. 4, p. 473.
- Partridge, John J.; Chadha, Naresh K.; Uskokovic, Milan R. (1990). "An asymmetric hydroboration of 5-substituted cyclopentadienes: synthesis of methyl (1R,5R)-5-hydroxy-2-cyclopentene-1-acetate". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 7, p. 339. - Girolami, G. S.; Rauchfuss, T. B. & Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. CA: University Science Books: Mill Valley. ISBN 0935702482.
- Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–84. Bibcode:1954JAChS..76.4281W. doi:10.1021/ja01646a008.
- Macomber, D. W.; Hart, W. P.; Rausch, M. D. (1982). "Functionally Substituted Cyclopentadienyl Metal Compounds". Adv. Organomet. Chem. Advances in Organometallic Chemistry. 21: 1–55. doi:10.1016/S0065-3055(08)60377-9. ISBN 9780120311217.
- Petrov, Alex R.; Jess, Kristof; Freytag, Matthias; Jones, Peter G.; Tamm, Matthias (2013). "Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives". Organometallics. 32 (20): 5946–5954. doi:10.1021/om4004972.
- Robert E. Dinnebier; Ulrich Behrens & Falk Olbrich (1997). "Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. Determination by High-Resolution Powder Diffraction". Organometallics. 16 (17): 3855–3858. doi:10.1021/om9700122.
- Elschenbroich, C. (2006). Organometallics. Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2.
- Reetz, Manfred T.; Hütte, Stephan; Goddard, Richard (1995-03-01). "Tetrabutylammonium Salts of 2-Nitropropane, Cyclopentadiene and 9-Ethylfluorene: Crystal Structures and Use in Anionic Polymerization". Zeitschrift für Naturforschung B. 50 (3): 415–422. doi:10.1515/znb-1995-0316. ISSN 1865-7117. S2CID 45791403.
| Salts and covalent derivatives of the cyclopentadienide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||