| Revision as of 19:18, 20 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - updated 'ChEBI_Ref', 'KEGG_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 19:34, 20 December 2023 edit undoJJMC89 bot III (talk | contribs)Bots, Administrators3,688,880 editsm Moving Category:Eli Lilly and Company brands to Category:Drugs developed by Eli Lilly and Company per Misplaced Pages:Categories for discussion/Log/2023 December 9#Category:AstraZeneca brands | ||
| (25 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 477855864 | ||
| | IUPAC_name = (9''S'')-9--6,7,10,11-tetrahydro-9''H'',18''H''-5,21:12,17-di(metheno)dibenzopyrrolooxadiazacyclohexadecine-18,20-dione | | IUPAC_name = (9''S'')-9--6,7,10,11-tetrahydro-9''H'',18''H''-5,21:12,17-di(metheno)dibenzopyrrolooxadiazacyclohexadecine-18,20-dione | ||
| | image = Ruboxistaurin.svg | | image = Ruboxistaurin.svg | ||
| | image2 = Ruboxistaurin 3D ball.png | |||
| | alt2 = Ball-and-stick model of the ruboxistaurin molecule | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = | | tradename = | ||
| Line 13: | Line 16: | ||
| | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| | legal_US = Not FDA approved | |||
| | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| | legal_status = | | legal_status = | ||
| | routes_of_administration = |
| routes_of_administration = | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| Line 22: | Line 25: | ||
| | metabolism = | | metabolism = | ||
| | elimination_half-life = | | elimination_half-life = | ||
| | excretion = |
| excretion = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | IUPHAR_ligand = 5263 | |||
| | CAS_number_Ref = {{cascite| |
| CAS_number_Ref = {{cascite|changed|??}} | ||
| | CAS_number = 169939-94-0 | | CAS_number = 169939-94-0 | ||
| | ATC_prefix = none | | ATC_prefix = none | ||
| Line 34: | Line 38: | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 135727 | | ChemSpiderID = 135727 | ||
| | UNII_Ref = {{fdacite| |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 721809WQCP | | UNII = 721809WQCP | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| Line 41: | Line 45: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=28 | H=28 | N=4 | O=3 | | C=28 | H=28 | N=4 | O=3 | ||
| | molecular_weight = 468.546 ]/] | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | | StdInChI = 1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = ZCBUQCWBWNUWSU-SFHVURJKSA-N | | StdInChIKey = ZCBUQCWBWNUWSU-SFHVURJKSA-N | ||
| |drug_name=|alt=|caption=|type=|MedlinePlus=|licence_EU=|licence_US=|SMILES=CN(C1()CCN2C=C(C(C(O)=NC3=O)=C3C(C4=CC=CC=C45)=CN5CCO1)C6=CC=CC=C62)C}} | |||
| }} | |||
| '''Ruboxistaurin''' (proposed brand name '''Arxxant''') is an investigational drug for diabetic |
'''Ruboxistaurin''' (proposed brand name '''Arxxant''') is an investigational drug for ] being investigated by ]. It is a member of the ] family. | ||
| In February 2006, Lilly submitted a ] for ruboxistaurin, and on August 18, 2006, Lilly received an ] from the ] for ruboxistaurin,<ref>{{cite web |url=https://www.drugs.com/nda/arxxant_060818.html |title=Drugs.com, Eli Lilly and Company Announces Approvable Letter Issued by FDA for Arxxant |accessdate=2008-02-15 }}</ref> with a request for an additional ], which would take 5 years to complete.<ref>{{cite web |url=https://www.drugs.com/nda/arxxant_060929.html |title=Drugs.com, Lilly Announces FDA Requirement of Additional Clinical Trial Before Ruboxistaurin Could Be Approved for Treatment of Diabetic Retinopathy |accessdate=2008-02-15 }}</ref> Lilly has not made any further request for approval and ruboxistaurin is not approved by the FDA for any medical use.<ref>{{Cite web | title= Arxxant Approval Status | url = https://www.drugs.com/history/arxxant.html | publisher = ] }}</ref> | |||
| ==Mechanism of action== | ==Mechanism of action== | ||
| Ruboxistaurin is an inhibitor of ]-beta.<ref>{{cite journal | |
Ruboxistaurin is an inhibitor of ]-beta.<ref>{{cite journal |vauthors=Clarke M, Dodson PM |title=PKC inhibition and diabetic microvascular complications |journal=Best Pract Res Clin Endocrinol Metab |volume=21 |issue=4 |pages=573–86 |date=December 2007 |pmid=18054736 |doi=10.1016/j.beem.2007.09.007}}</ref> | ||
| ==References== | ==References== | ||
| Line 58: | Line 61: | ||
| ==External links== | ==External links== | ||
| * | * | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 19:34, 20 December 2023
Chemical compound Pharmaceutical compound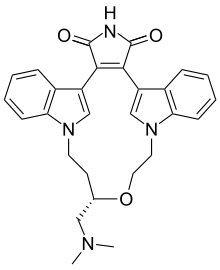 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H28N4O3 |
| Molar mass | 468.557 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Ruboxistaurin (proposed brand name Arxxant) is an investigational drug for diabetic retinopathy being investigated by Eli Lilly and Company. It is a member of the bisindolylmaleimide family.
In February 2006, Lilly submitted a New Drug Application for ruboxistaurin, and on August 18, 2006, Lilly received an approvable letter from the US FDA for ruboxistaurin, with a request for an additional clinical trial, which would take 5 years to complete. Lilly has not made any further request for approval and ruboxistaurin is not approved by the FDA for any medical use.
Mechanism of action
Ruboxistaurin is an inhibitor of protein kinase C-beta.
References
- "Drugs.com, Eli Lilly and Company Announces Approvable Letter Issued by FDA for Arxxant". Retrieved 2008-02-15.
- "Drugs.com, Lilly Announces FDA Requirement of Additional Clinical Trial Before Ruboxistaurin Could Be Approved for Treatment of Diabetic Retinopathy". Retrieved 2008-02-15.
- "Arxxant Approval Status". drugs.com.
- Clarke M, Dodson PM (December 2007). "PKC inhibition and diabetic microvascular complications". Best Pract Res Clin Endocrinol Metab. 21 (4): 573–86. doi:10.1016/j.beem.2007.09.007. PMID 18054736.
External links
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |