| Revision as of 14:29, 21 October 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL', 'CASNo').← Previous edit | Latest revision as of 03:20, 22 January 2024 edit undoEejit43Bot (talk | contribs)Bots9,175 editsm →See also: Fix improperly capitalized section headers, general fixesTag: AWB | ||
| (28 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 456683340 | ||
| | Name = | | Name = | ||
| | ImageFile = Amarogentin.png | | ImageFile = Amarogentin.png | ||
| Line 6: | Line 8: | ||
| | ImageName = Chemical structure of amarogentin | | ImageName = Chemical structure of amarogentin | ||
| | ImageAlt = Chemical structure of amarogentin | | ImageAlt = Chemical structure of amarogentin | ||
| | ImageCaption = Chemical structure of amarogentin | |||
| | ImageFile2 = Amarogentin 3D BS.png | |||
| ⚫ | | |
||
| | ImageSize2 = 200px | |||
| | ImageName2 = Chemical structure of amarogentin | |||
| | ImageAlt2 = Chemical structure of amarogentin | |||
| | ImageCaption2 = Chemical structure of amarogentin | |||
| | IUPACName = (4a''S'',5''R'',6''S'')-5-Ethenyl-1-oxo-4,4a,5,6-tetrahydro-1''H'',3''H''-pyranopyran-6-yl β-<small>D</small>-glucopyranoside 2-(3,3′,5-trihydroxy-2-carboxylate) | |||
| ⚫ | | SystematicName = (2''S'',3''R'',4''S'',5''S'',6''R'')-2-{pyran-6-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,3′,5-trihydroxy-2-carboxylate | ||
| | OtherNames = <!-- <br> --> | | OtherNames = <!-- <br> --> | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | CASNo = |
| CASNo = 21018-84-8 | ||
| | CASNo_Ref = | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| CASNoOther = | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 5L82GT5I0W | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 451112 | | ChEMBL = 451112 | ||
| | PubChem = 115149 | | PubChem = 115149 | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 103033 | | ChemSpiderID = 103033 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 2622 | | ChEBI = 2622 | ||
| | SMILES = O=C/1OCC5C\1=C\O(O4O((O)(O)4OC(=O)c3c(c2cccc(O)c2)cc(O)cc3O)CO)5\C=C | | SMILES = O=C/1OCC5C\1=C\O(O4O((O)(O)4OC(=O)c3c(c2cccc(O)c2)cc(O)cc3O)CO)5\C=C | ||
| | |
| InChI = 1/C29H30O13/c1-2-16-17-6-7-38-26(36)19(17)12-39-28(16)42-29-25(24(35)23(34)21(11-30)40-29)41-27(37)22-18(9-15(32)10-20(22)33)13-4-3-5-14(31)8-13/h2-5,8-10,12,16-17,21,23-25,28-35H,1,6-7,11H2/t16-,17+,21-,23-,24+,25-,28+,29+/m1/s1 | ||
| | |
| InChIKey = DBOVHQOUSDWAPQ-WTONXPSSBG | ||
| | |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C29H30O13/c1-2-16-17-6-7-38-26(36)19(17)12-39-28(16)42-29-25(24(35)23(34)21(11-30)40-29)41-27(37)22-18(9-15(32)10-20(22)33)13-4-3-5-14(31)8-13/h2-5,8-10,12,16-17,21,23-25,28-35H,1,6-7,11H2/t16-,17+,21-,23-,24+,25-,28+,29+/m1/s1 | | StdInChI = 1S/C29H30O13/c1-2-16-17-6-7-38-26(36)19(17)12-39-28(16)42-29-25(24(35)23(34)21(11-30)40-29)41-27(37)22-18(9-15(32)10-20(22)33)13-4-3-5-14(31)8-13/h2-5,8-10,12,16-17,21,23-25,28-35H,1,6-7,11H2/t16-,17+,21-,23-,24+,25-,28+,29+/m1/s1 | ||
| | |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = DBOVHQOUSDWAPQ-WTONXPSSSA-N | | StdInChIKey = DBOVHQOUSDWAPQ-WTONXPSSSA-N | ||
| | MeSHName = | | MeSHName = | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| C=29 | H=30 | O=13 | ||
| | MolarMass = 586.54 g/mol | |||
| | ExactMass = 586.168641 u | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| | MeltingPt = |
| MeltingPt = | ||
| | BoilingPt = |
| BoilingPt = | ||
| | Solubility = | | Solubility = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | GHSPictograms = | |||
| | RPhrases = <!-- {{R10}}, {{R23}}, {{R34}}, {{R50}} etc. --> | |||
| | GHSSignalWord = | |||
| | SPhrases = <!-- {{S1/2}}, {{S9}}, {{S16}}, {{S26}}, {{S36/37/39}}, {{S45}}, {{S61}} etc. --> | |||
| | HPhrases = {{HPhrases|}} | |||
| | PPhrases = {{PPhrases|}} | |||
| | GHS_ref = <ref>GHS: "Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008"</ref> | |||
| }} | }} | ||
| }} | }} | ||
| ⚫ | '''Amarogentin''' is a chemical compound found in gentian ('']'') or in '']''.<ref>Production of |
||
| ⚫ | '''Amarogentin''' is a chemical compound found in gentian ('']'') or in '']''.<ref>{{cite journal | doi = 10.1055/s-2000-8579 | title = Production of Amarogentin in Root Cultures of Swertia chirata | year = 2000 | last1 = Keil* | first1 = Michael | last2 = Härtle | first2 = Birgit | last3 = Guillaume | first3 = Anna | last4 = Psiorz | first4 = Manfred | journal = Planta Medica | volume = 66 | issue = 5 | pages = 452–7 | pmid = 10909267| s2cid = 21149742 }}</ref> | ||
| ⚫ | ] | ||
| ⚫ | Gentian root has a long history of use as a herbal bitter in the treatment of digestive disorders and is an ingredient of many proprietary medicines. The bitter principles of gentian root are |
||
| ⚫ | ]{{clear left}} | ||
| It also shows an ] activity<ref>Evaluation of the in-vivo activity and toxicity of amarogentin, an antileishmanial agent, in both liposomal and niosomal forms. Swapna Medda, Sibabrata Mukhopadhyay and Mukul Kumar Basu, J. Antimicrob. Chemother., (1999) 44 (6), pages 791-794, {{doi|10.1093/jac/44.6.791}}</ref> being an ].<ref>Amarogentin, a Naturally Occurring Secoiridoid Glycoside and a Newly Recognized Inhibitor of Topoisomerase I from Leishmania donovani. Sutapa Ray, Hemanta K. Majumder, Ajit K. Chakravarty, and Sibabrata Mukhopadhyay, J. Nat. Prod., 1996, 59 (1), pages 27–29, {{doi|10.1021/np960018g}}</ref> | |||
| ⚫ | Gentian root has a long history of use as a herbal bitter in the treatment of digestive disorders and is an ingredient of many proprietary medicines. The bitter principles of gentian root are ] ]s amarogentin and ]. The former is one of the most ] natural compounds known<ref> {{Webarchive|url=https://web.archive.org/web/20090902132313/http://www.awl.ch/heilpflanzen/gentiana_lutea/index.htm |date=2009-09-02 }} ('''German''')</ref> and is used as a scientific basis for measuring bitterness. In humans, it activates the bitter ] ].<ref>{{cite journal | doi = 10.1021/jf9014334 | title = The Human Bitter Taste Receptor hTAS2R50 is Activated by the Two Natural Bitter Terpenoids Andrographolide and Amarogentin | year = 2009 | last1 = Behrens | first1 = Maik | last2 = Brockhoff | first2 = Anne | last3 = Batram | first3 = Claudia | last4 = Kuhn | first4 = Christina | last5 = Appendino | first5 = Giovanni | last6 = Meyerhof | first6 = Wolfgang | journal = Journal of Agricultural and Food Chemistry | volume = 57 | issue = 21 | pages = 9860–6 | pmid = 19817411}}</ref> The biphenylcarboxylic acid moiety is biosynthesized by a ]-type pathway, with three units of ] and one unit of ], this being formed from an early ] intermediate and not via cinnamic or benzoic acid.<ref>{{cite journal | doi = 10.1002/1099-0690(200104)2001:8<1459::AID-EJOC1459>3.0.CO;2-0 | title = Unexpected Biosynthetic Precursors of Amarogentin − A Retrobiosynthetic13C NMR Study | year = 2001 | last1 = Wang | first1 = Chang-Zeng | last2 = Maier | first2 = Ulrich H. | last3 = Eisenreich | first3 = Wolfgang | last4 = Adam | first4 = Petra | last5 = Obersteiner | first5 = Ingrid | last6 = Keil | first6 = Michael | last7 = Bacher | first7 = Adelbert | last8 = Zenk | first8 = Meinhart H. | journal = European Journal of Organic Chemistry | volume = 2001 | issue = 8 | pages = 1459}}</ref> | ||
| It also shows an ] activity in ]s<ref>{{cite journal | doi = 10.1093/jac/44.6.791 | title = Evaluation of the in-vivo activity and toxicity of amarogentin, an antileishmanial agent, in both liposomal and niosomal forms | year = 1999 | last1 = Medda | first1 = S. | journal = Journal of Antimicrobial Chemotherapy | volume = 44 | issue = 6 | pages = 791–4 | pmid = 10590280 | last2 = Mukhopadhyay | first2 = S | last3 = Basu | first3 = MK| doi-access = free }}</ref> being an ].<ref>{{cite journal | doi = 10.1021/np960018g | title = Amarogentin, a Naturally Occurring Secoiridoid Glycoside and a Newly Recognized Inhibitor of Topoisomerase I fromLeishmania donovani | year = 1996 | last1 = Ray | first1 = Sutapa | last2 = Majumder | first2 = Hemanta K. | last3 = Chakravarty | first3 = Ajit K. | last4 = Mukhopadhyay | first4 = Sibabrata | last5 = Gil | first5 = Roberto R. | last6 = Cordell | first6 = Geoffrey A. | journal = Journal of Natural Products | volume = 59 | pages = 27–9 | pmid = 8984149 | issue = 1}}</ref> | |||
| ==See also== | |||
| * ] | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist|2}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| {{natural-phenol-stub}} | |||
Latest revision as of 03:20, 22 January 2024
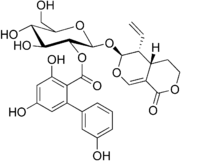 Chemical structure of amarogentin | |
 Chemical structure of amarogentin | |
| Names | |
|---|---|
| IUPAC name (4aS,5R,6S)-5-Ethenyl-1-oxo-4,4a,5,6-tetrahydro-1H,3H-pyranopyran-6-yl β-D-glucopyranoside 2-(3,3′,5-trihydroxy-2-carboxylate) | |
| Systematic IUPAC name (2S,3R,4S,5S,6R)-2-{pyran-6-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,3′,5-trihydroxy-2-carboxylate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.166.688 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C29H30O13 |
| Molar mass | 586.546 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Amarogentin is a chemical compound found in gentian (Gentiana lutea) or in Swertia chirata.
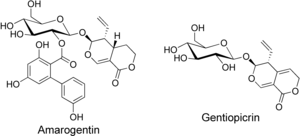
Gentian root has a long history of use as a herbal bitter in the treatment of digestive disorders and is an ingredient of many proprietary medicines. The bitter principles of gentian root are secoiridoid glycosides amarogentin and gentiopicrin. The former is one of the most bitter natural compounds known and is used as a scientific basis for measuring bitterness. In humans, it activates the bitter taste receptor TAS2R50. The biphenylcarboxylic acid moiety is biosynthesized by a polyketide-type pathway, with three units of acetyl-CoA and one unit of 3-hydroxybenzoyl-CoA, this being formed from an early shikimate pathway intermediate and not via cinnamic or benzoic acid.
It also shows an antileishmanial activity in animal models being an inhibitor of topoisomerase I.
See also
References
- Keil*, Michael; Härtle, Birgit; Guillaume, Anna; Psiorz, Manfred (2000). "Production of Amarogentin in Root Cultures of Swertia chirata". Planta Medica. 66 (5): 452–7. doi:10.1055/s-2000-8579. PMID 10909267. S2CID 21149742.
- Heilpflanzen:Gentiana lutea Archived 2009-09-02 at the Wayback Machine (German)
- Behrens, Maik; Brockhoff, Anne; Batram, Claudia; Kuhn, Christina; Appendino, Giovanni; Meyerhof, Wolfgang (2009). "The Human Bitter Taste Receptor hTAS2R50 is Activated by the Two Natural Bitter Terpenoids Andrographolide and Amarogentin". Journal of Agricultural and Food Chemistry. 57 (21): 9860–6. doi:10.1021/jf9014334. PMID 19817411.
- Wang, Chang-Zeng; Maier, Ulrich H.; Eisenreich, Wolfgang; Adam, Petra; Obersteiner, Ingrid; Keil, Michael; Bacher, Adelbert; Zenk, Meinhart H. (2001). "Unexpected Biosynthetic Precursors of Amarogentin − A Retrobiosynthetic13C NMR Study". European Journal of Organic Chemistry. 2001 (8): 1459. doi:10.1002/1099-0690(200104)2001:8<1459::AID-EJOC1459>3.0.CO;2-0.
- Medda, S.; Mukhopadhyay, S; Basu, MK (1999). "Evaluation of the in-vivo activity and toxicity of amarogentin, an antileishmanial agent, in both liposomal and niosomal forms". Journal of Antimicrobial Chemotherapy. 44 (6): 791–4. doi:10.1093/jac/44.6.791. PMID 10590280.
- Ray, Sutapa; Majumder, Hemanta K.; Chakravarty, Ajit K.; Mukhopadhyay, Sibabrata; Gil, Roberto R.; Cordell, Geoffrey A. (1996). "Amarogentin, a Naturally Occurring Secoiridoid Glycoside and a Newly Recognized Inhibitor of Topoisomerase I fromLeishmania donovani". Journal of Natural Products. 59 (1): 27–9. doi:10.1021/np960018g. PMID 8984149.