| Revision as of 12:02, 24 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to verified fields - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|errors...← Previous edit | Latest revision as of 09:07, 9 September 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,126 edits removed Category:Chlorobenzene derivatives; added Category:4-Chlorophenyl compounds using HotCat | ||
| (43 intermediate revisions by 25 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 477223140 | ||
| | |
| Name = 4-nitrochlorobenzene | ||
| | |
| ImageFileL1 = 4-chloronitrobenzene.png | ||
| | ImageSize = 150px | |||
| | ImageSizeL1 = 145 | |||
| ⚫ | | |
||
| | ImageAltL1 = Skeletal formula of 4-nitrochlorobenzene | |||
| ⚫ | | |
||
| | ImageFileR1 = 4-Nitrochlorobenzene-3D-balls.png | |||
| ⚫ | | |
||
| | ImageSizeR1 = 105 | |||
| ⚫ | | |
||
| | ImageAltR1 = Ball-and-stick model of the 4-nitrochlorobenzene molecule | |||
| | CASNo = 100-00-5 | |||
| ⚫ | | PIN = 1-Chloro-4-nitrobenzene | ||
| ⚫ | | |
||
| ⚫ | | OtherNames = 4-Chloro-1-nitrobenzene<br />4-Chloronitrobenzene<br />''p''-Nitrochlorobenzene<br />PNCBO | ||
| ⚫ | | |
||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID = |
||
| | |
| CASNo = 100-00-5 | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | |
||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | UNII = CVL66U249D | |||
| | PubChem = 7474 | |||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | ||
| ⚫ | | ChemSpiderID = 21106020 | ||
| ⚫ | | SMILES = C1=CC(=CC=C1(=O))Cl | ||
| | InChI = 1/C6H4ClNO2/c7-5-1-3-6(4-2-5)8(9)10/h1-4H | |||
| | InChIKey = CZGCEKJOLUNIFY-UHFFFAOYAO | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C6H4ClNO2/c7-5-1-3-6(4-2-5)8(9)10/h1-4H | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = CZGCEKJOLUNIFY-UHFFFAOYSA-N | |||
| ⚫ | | RTECS = | ||
| | KEGG_Ref = {{keggcite|changed|kegg}} | | KEGG_Ref = {{keggcite|changed|kegg}} | ||
| | KEGG = C14456 | | KEGG = C14456 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=6 | H=4 | O=2 | N=1 | Cl=1 | ||
| | |
| Appearance = Light yellow solid | ||
| | Odor = sweet<ref name=PGCH/> | |||
| | |
| Density = 1.52 g/cm<sup>3</sup> (20 °C) | ||
| | |
| Solubility = Insoluble | ||
| | |
| SolubleOther = Soluble in toluene, ether, acetone, hot ethanol | ||
| | |
| Solvent = other solvents | ||
| | MeltingPtC = 83.6 | |||
| | |
| MeltingPtC = 83.6 | ||
| | |
| BoilingPtC = 242.0 | ||
| | Viscosity = | | pKa = | ||
| | Viscosity = | |||
| | |
| Dipole = | ||
| | |
| RefractIndex = | ||
| | VaporPressure = 0.2 mmHg (30°C)<ref name=PGCH/> | |||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | |
| ExternalSDS = | ||
| | |
| MainHazards = | ||
| | |
| NFPA-H = | ||
| | |
| NFPA-F = | ||
| | |
| NFPA-R = | ||
| | |
| AutoignitionPtC = | ||
| | |
| FlashPtC = 12 | ||
| | LD50 = 812 mg/kg (rat, oral)<br/>1414 mg/kg (mouse, oral)<br/>440 mg/kg (mouse, oral)<br/>420 mg/kg (rat, oral)<ref name=PGCH/> | |||
| | NFPA-R = | |||
| | PEL = TWA 1 mg/m<sup>3</sup> <ref name=PGCH>{{PGCH|0452}}</ref> | |||
| | Autoignition = | |||
| | IDLH = Ca <ref name=PGCH/> | |||
| | FlashPt = | |||
| | LC50 = 164 mg/m<sup>3</sup> (cat, 7 hr)<ref name=PGCH/> | |||
| | LD50 = | |||
| | REL = Ca<ref name=PGCH/> | |||
| }} | }} | ||
| }} | }} | ||
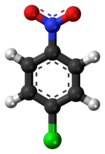
| '''4-Nitrochlorobenzene''' is the ] with the formula ClC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub>. |
'''4-Nitrochlorobenzene''' is the ] with the formula ClC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub>. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including ]s commonly found in ]. Other ]s with the formula ClC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> include ] and 3-nitrochlorobenzene. | ||
| ==Preparation== | ==Preparation== | ||
| 4-Nitrochlorobenzene is prepared industrially by nitration of ]: | |||
| ⚫ | 4-Nitrochlorobenzene was originally prepared by the ] of 4 |
||
| :{{chem2|ClC6H5 + HNO3 -> ClC6H4NO2 + H2O}} | |||
| ⚫ | This reaction affords both the 2- and the 4-nitro derivatives, in about a 1:2 ratio. These isomers are separated by a combination of crystallization and distillation.<ref name=Ullmann/> 4-Nitrochlorobenzene was originally prepared by the ] of 4-bromochlorobenzene by Holleman and coworkers.<ref>"The nitration of mixed dihalogen benzenes" Recueil des Travaux Chimiques des Pays-Bas et de la Belgique. Amsterdam, 1915; pp. 204-235.</ref> | ||
| :] | |||
| ⚫ | ==Applications== | ||
| Currently, 4-nitrochlorobenzene is prepared on an industrial scale from chlorobenzene via ] using ] or ] catalysts:<ref>Zhang, Cun; Liu, Tao; Ma, Chunyan. U.S. Patent 10,235,242, 2008.</ref> | |||
| 4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives. ] gives ], and ]. Reduction with iron metal gives ]. The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to ], unlike related chlorobenzene. Thus, the strong ]s hydroxide, ], ], and ] displace chloride to give respectively ], 4-nitroanisole, ], and ].<ref name=Ullmann>{{cite encyclopedia|author=Gerald Booth|title=Nitro Compounds, Aromatic|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|year=2007|publisher=Wiley-VCH|location=Weinheim|doi=10.1002/14356007.a17_411|isbn=978-3527306732}}</ref><ref>{{cite journal |doi=10.15227/orgsyn.014.0066 |title=p-Nitrodiphenyl Ether |journal=Organic Syntheses |year=1934 |volume=14 |page=66|first1=Ray Q.|last1=Brewster|first2=Theodore |last2=Groening }}</ref> | |||
| ⚫ | Another use of 4-nitrochlorobenzene is its condensation with aniline to produce 4-nitrodiphenylamine. Reductive alkylation of the nitro group affords secondary aryl amines, which are useful antioxidants for rubber. | ||
| :] | |||
| ] drug ] (4-aniline).<ref name=Ullmann/>]] | |||
| ⚫ | ==Applications== | ||
| {{clear left}} | |||
| 4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives, including ], ], ], ], ], and ].<ref name=Ullmann>Nitro Compounds, Aromatic. ''Ullmann's Encyclopedia of Industrial Chemistry'', 7th Ed.; Wiley & Sons: New York, 1997; pp. 18-19</ref> These reactions mainly involve the nucleophilic displacement of chloride . The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to ], unlike related chlorobenzene. | |||
| ==Safety== | |||
| ⚫ | Another |
||
| The U.S. ] considers 4-nitrochlorobenzene as a potential occupational carcinogen.<ref></ref> The ] set a permissible exposure limit of 1 mg/m<sup>3</sup> The ] recommends an airborne exposure limit of 0.64 mg/m<sup>3</sup> over a time-weighted average of eight hours.<ref></ref><ref></ref> | |||
| :] | |||
| ==References== | ==References== | ||
| Line 69: | Line 86: | ||
| {{DEFAULTSORT:Nitrochlorobenzene, 4-}} | {{DEFAULTSORT:Nitrochlorobenzene, 4-}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 09:07, 9 September 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1-Chloro-4-nitrobenzene | |||
| Other names
4-Chloro-1-nitrobenzene 4-Chloronitrobenzene p-Nitrochlorobenzene PNCBO | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.554 | ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H4ClNO2 | ||
| Molar mass | 157.55 g·mol | ||
| Appearance | Light yellow solid | ||
| Odor | sweet | ||
| Density | 1.52 g/cm (20 °C) | ||
| Melting point | 83.6 °C (182.5 °F; 356.8 K) | ||
| Boiling point | 242.0 °C (467.6 °F; 515.1 K) | ||
| Solubility in water | Insoluble | ||
| Solubility in other solvents | Soluble in toluene, ether, acetone, hot ethanol | ||
| Vapor pressure | 0.2 mmHg (30°C) | ||
| Hazards | |||
| Flash point | 12 °C (54 °F; 285 K) | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 812 mg/kg (rat, oral) 1414 mg/kg (mouse, oral) 440 mg/kg (mouse, oral) 420 mg/kg (rat, oral) | ||
| LC50 (median concentration) | 164 mg/m (cat, 7 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 1 mg/m | ||
| REL (Recommended) | Ca | ||
| IDLH (Immediate danger) | Ca | ||
| Safety data sheet (SDS) | External MSDS | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including antioxidants commonly found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.
Preparation
4-Nitrochlorobenzene is prepared industrially by nitration of chlorobenzene:
- ClC6H5 + HNO3 → ClC6H4NO2 + H2O
This reaction affords both the 2- and the 4-nitro derivatives, in about a 1:2 ratio. These isomers are separated by a combination of crystallization and distillation. 4-Nitrochlorobenzene was originally prepared by the nitration of 4-bromochlorobenzene by Holleman and coworkers.
Applications
4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives. Nitration gives 2,4-dinitrochlorobenzene, and 3,4-dichloronitrobenzene. Reduction with iron metal gives 4-chloroaniline. The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to nucleophilic aromatic substitution, unlike related chlorobenzene. Thus, the strong nucleophiles hydroxide, methoxide, fluoride, and amide displace chloride to give respectively 4-nitrophenol, 4-nitroanisole, 4-fluoronitrobenzene, and 4-nitroaniline.
Another use of 4-nitrochlorobenzene is its condensation with aniline to produce 4-nitrodiphenylamine. Reductive alkylation of the nitro group affords secondary aryl amines, which are useful antioxidants for rubber.
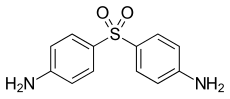
Safety
The U.S. National Institute for Occupational Safety and Health considers 4-nitrochlorobenzene as a potential occupational carcinogen. The Occupational Safety and Health Administration set a permissible exposure limit of 1 mg/m The American Conference of Governmental Industrial Hygienists recommends an airborne exposure limit of 0.64 mg/m over a time-weighted average of eight hours.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0452". National Institute for Occupational Safety and Health (NIOSH).
- ^ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- "The nitration of mixed dihalogen benzenes" Recueil des Travaux Chimiques des Pays-Bas et de la Belgique. Amsterdam, 1915; pp. 204-235.
- Brewster, Ray Q.; Groening, Theodore (1934). "p-Nitrodiphenyl Ether". Organic Syntheses. 14: 66. doi:10.15227/orgsyn.014.0066.
- CDC - Immediately Dangerous to Life or Health Concentrations (IDLH): p-nitrochlorobenzene
- CDC - NIOSH Pocket Guide to Chemical Hazards
- New Jersey Department of Health and Senior Services - Hazardous Substance Fact Sheet