| Revision as of 06:53, 8 November 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[user talk:Che...← Previous edit | Latest revision as of 17:10, 4 January 2025 edit undoCitation bot (talk | contribs)Bots5,457,781 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Pancho507 | Linked from User:Pancho507/sandbox/1 | #UCB_webform_linked 25/3849 | ||
| (83 intermediate revisions by 50 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 459589749 | | verifiedrevid = 459589749 | ||
| |ImageFile=Benzyl-cyanide.png | | ImageFile =Benzyl-cyanide.png | ||
| |ImageSize=100px | | ImageSize =100px | ||
| | ImageAlt = Skeletal formula of benzyl cyanide | |||
| |IUPACName=2-Phenylacetonitrile | |||
| | ImageFile1 = Benzyl-cyanide-3D-balls.png | |||
| |OtherNames= phenylacetonitrile, Éø-tolunitrile | |||
| | ImageSize1 = 160 | |||
| ⚫ | |Section1= |
||
| | ImageAlt1 = Ball-and-stick model of the benzyl cyanide molecule | |||
| ⚫ | | |
||
| | PIN = Phenylacetonitrile<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = ] | date = 2014 | location = Cambridge | page = 16 | doi = 10.1039/9781849733069-FP001 | isbn = 978-0-85404-182-4}}</ref> | |||
| | OtherNames = Benzyl cyanide<ref name=iupac2013 /><br />2-Phenylacetonitrile<br />α-Tolunitrile<br />Benzylnitrile | |||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C16074 | | KEGG = C16074 | ||
| | InChI = 1/C8H7N/c9-7-6-8-4-2-1-3-5-8/h1-5H,6H2 | | InChI = 1/C8H7N/c9-7-6-8-4-2-1-3-5-8/h1-5H,6H2 | ||
| | InChIKey = SUSQOBVLVYHIEX-UHFFFAOYAJ | | InChIKey = SUSQOBVLVYHIEX-UHFFFAOYAJ | ||
| | SMILES = N#CCc1ccccc1 | |||
| | SMILES1 = c1ccc(cc1)CC#N | |||
| | StdInChI = 1S/C8H7N/c9-7-6-8-4-2-1-3-5-8/h1-5H,6H2 | | StdInChI = 1S/C8H7N/c9-7-6-8-4-2-1-3-5-8/h1-5H,6H2 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 16: | Line 21: | ||
| | StdInChIKey = SUSQOBVLVYHIEX-UHFFFAOYSA-N | | StdInChIKey = SUSQOBVLVYHIEX-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo=140-29-4 | | CASNo =140-29-4 | ||
| | |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 23G40PRP93 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 25979 | | ChEBI = 25979 | ||
| | PubChem=8794 | | PubChem =8794 | ||
| | SMILES=C1=CC=C(C=C1)CC#N | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 13839308 | | ChemSpiderID = 13839308 | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| Formula =C<sub>8</sub>H<sub>7</sub>N | ||
| | |
| MolarMass =117.15 g/mol | ||
| | |
| Appearance =Colorless oily liquid | ||
| | |
| Density =1.015 g/cm<sup>3</sup> | ||
| | |
| MeltingPtC = -24 | ||
| | MeltingPt_notes = | |||
| | BoilingPt=233-234 °C | |||
| | BoilingPtC = 233 to 234 | |||
| | Solubility= | |||
| | BoilingPt_notes = | |||
| ⚫ | |||
| | Solubility = | |||
| ⚫ | |Section3= |
||
| | MagSus = -76.87·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| | MainHazards= | |||
| ⚫ | }} | ||
| | FlashPt= | |||
| ⚫ | |Section3={{Chembox Hazards | ||
| | Autoignition= | |||
| | MainHazards = | |||
| ⚫ | |||
| | FlashPt = | |||
| | AutoignitionPt = | |||
| ⚫ | }} | ||
| }} | }} | ||
| '''Benzyl cyanide''' (abbreviated '''BnCN''') is an ] with the ] C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>CN. This colorless oily aromatic liquid is |
'''Benzyl cyanide''' (abbreviated '''BnCN''') is an ] with the ] C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>CN. This colorless oily aromatic liquid is an important ] to numerous compounds in ].<ref name=Ullmann>{{cite journal|last1=Pollak|first1=Peter|last2=Romeder|first2=Gérard|last3=Hagedorn|first3=Ferdinand|last4=Gelbke|first4=Heinz-Peter|title=Nitriles|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|doi=10.1002/14356007.a17_363|isbn=3527306730}}</ref> | ||
| It is also an important ] in certain species.<ref>{{cite news|title=Toxin Protects Migratory Locusts from Cannibalism|date=4 May 2023|url=https://www.mpg.de/20278575/0504-choe-chemisches-signal-schuetzt-wanderheuschrecken-vor-kannibalismus|publisher=]|access-date=8 December 2024}}</ref> | |||
| ==Preparation== | |||
| ==Synthesis, reactions, and applications== | |||
| Benzyl cyanide can be produced via ] between ] and ]<ref>{{cite journal|last1=Adams|first1=Roger|last2=Thal|first2=A. F.|title=Benzyl cyanide|journal=Organic Syntheses|date=1922|volume=2|page=9|doi=10.15227/orgsyn.002.0009}}</ref> and by ] of ].<ref>{{cite journal|last1=Hiegel|first1=Gene|last2=Lewis|first2=Justin|last3=Bae|first3=Jason|title=Conversion of α-Amino Acids into Nitriles by Oxidative Decarboxylation with Trichloroisocyanuric Acid|journal=Synthetic Communications|date=2004|volume=34|issue=19|pages=3449–3453|doi=10.1081/SCC-200030958|s2cid=52208189}}</ref> | |||
| Benzyl cyanide is produced by the reaction of ] with ]. | |||
| Benzyl cyanides can also be prepared by arylation of silyl-substituted acetonitrile.<ref>{{cite journal |doi=10.1021/ja053027x|title=Mild Palladium-Catalyzed Selective Monoarylation of Nitriles|year=2005|last1=Wu|first1=Lingyun|last2=Hartwig|first2=John F.|journal=Journal of the American Chemical Society|volume=127|issue=45|pages=15824–15832|pmid=16277525|bibcode=2005JAChS.12715824W }}</ref> | |||
| The compound's most important reactions involve the "active ] group," which is readily functionalized following deprotonation.<ref>{{OrgSynth | collvol = 6 | collvolpages = 199 | year= 1988 | title = New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile | authors = Masumi Itoh, Daijiro Hagiwara, Takashi Kamiya | prep = cv6p0199}}</ref><ref>{{OrgSynth | collvol = 3 | collvolpages = 715 | year= 1955 | title = α-Phenylcinnamonitrile | authors = Stanley Wawzonek, Edwin M. Smolin | prep = cv3p0715}}</ref> | |||
| ==Reactions== | |||
| BnCN it is an important intermediate for a variety of useful – and some dangerous - compounds. It is used in the production of ], ], and other ]. For this reason it is on the U.S.'s ]. It is also used to make ] and also pethidine and ketobemidone (if further substituted). | |||
| Benzyl cyanide undergoes many reactions characteristic of nitriles. It can be ] to give ]<ref>{{cite journal|last1=Adams|first1=Roger|last2=Thal|first2=A. F.|title=Phenylacetic acid|journal=Organic Syntheses|date=1922|volume=2|page=59|doi=10.15227/orgsyn.002.0059}}</ref> or it can be used in the ] to yield phenylacetic acid ].<ref>{{cite journal|last1=Adams|first1=Roger|last2=Thal|first2=A. F.|title=Ethyl Phenylacetate|journal=Organic Syntheses|date=1922|volume=2|page=27|doi=10.15227/orgsyn.002.0027}}</ref> Hydrogenation gives ].<ref>{{cite journal |doi=10.15227/orgsyn.023.0071|title=β-Phenylethylamine|journal=Organic Syntheses|year=1943|volume=23|page=71|first1=John C. Jr.|last1=Robinson|first2=H. R.|last2=Snyder}}</ref> | |||
| The compound contains an "active ]". Bromination occurs gives PhCHBrCN.<ref>{{cite journal |first1=C. M.|last1=Robb|first2=E. M.|last2=Schultz|doi=10.15227/orgsyn.028.0055|title=Diphenylacetonitrile|journal=Organic Syntheses|year=1948|volume=28|page=55}}</ref> A variety of base-induced reactions result in the formation of new ]s.<ref>{{cite journal|last1=Makosza|first1=M.|last2=Jonczyk|first2=A|title=Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile|journal=Organic Syntheses|date=1976|volume=55|page=91|doi=10.15227/orgsyn.055.0091}}</ref><ref>{{cite journal|last1=Itoh|first1=Masumi|last2=Hagiwara|first2=Daijiro|last3=Kamiya|first3=Takashi|title=New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile|journal=Organic Syntheses|date=1988|volume=6|page=199|doi=10.15227/orgsyn.059.0095}}</ref><ref>{{cite journal|last1=Wawzonek|first1=Stanley|last2=Smolin|first2=Edwin M.|title=α-Phenylcinnamonitrile|journal=Organic Syntheses|date=1955|volume=3|page=715|doi=10.15227/orgsyn.029.0083}}</ref> | |||
| BnCN is the precursor to ] and related compounds. For example, BnCN reacts with 1,3-dihalopropane in the presence of a strong base to form the cyclobutane precursor to ]. One route to ] involves ] of the ] compound. The Clemmensen reduction is avoided if the synthesis starts with ] of BnCN followed by hydrolysis and reductive amination. | |||
| ==Uses== | |||
| Benzyl cyanide is used as a solvent<ref>{{cite journal|last1=Bien|first1=Hans-Samuel|last2=Stawitz|first2=Josef|last3=Wunderlich|first3=Klaus|title=Anthraquinone Dyes and Intermediates|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|page=29|doi=10.1002/14356007.a02_355|isbn=3527306730}}</ref> and as a starting material in the synthesis of ]s (e.g. ]),<ref>{{cite journal|last1=Ackermann|first1=Peter|last2=Margot|first2=Paul|last3=Müller|first3=Franz|title=Fungicides, Agricultural|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|doi=10.1002/14356007.a12_085|isbn=3527306730}}</ref> fragrances (]), ]s,<ref name=Ullmann /> and other ]. The partial hydrolysis of BnCN results in ].<ref>{{cite journal|title=PHENYLACETAMIDE|journal=Organic Syntheses|volume=32|year=1952|pages=92|issn=0078-6209|doi=10.15227/orgsyn.032.0092}}</ref> | |||
| ===Pharmaceuticals=== | |||
| Benzyl cyanide is a useful ] to numerous pharmaceuticals. Examples include: | |||
| <ref name=William>{{cite book|last1=William Andrew Publishing|title=Pharmaceutical Manufacturing Encyclopedia|date=2008|publisher=Elsevier Science|location=Norwich, NY|isbn=9780815515265|pages=182, 936, 1362, 1369, 1505, 2036, 2157, 2259, 2554, 2620, 2660, 2670, 2924, 3032, & 3410|edition=3rd|url=https://books.google.com/books?id=bRX8MwEACAAJ&q=9780815515265}}</ref> | |||
| *]s (e.g. ])<ref name=William /> | |||
| *Antidepressants: E.g. ] & ] | |||
| *]s (e.g. ] (para-fluoro),<ref name=William /><ref>{{cite journal|last1=Berkoff|first1=Charles E.|last2=Rivard|first2=Donald E.|last3=Kirkpatrick|first3=David|last4=Ives|first4=Jeffrey L.|title=The Reductive Decyanation of Nitriles by Alkali Fusion|journal=Synthetic Communications|date=1980|volume=10|issue=12|pages=939–945|doi=10.1080/00397918008061855}}</ref> ] & ]. | |||
| *] (e.g. ], ], ], ], and ])<ref>{{cite journal|last1=Bub|first1=Oskar|last2=Friedrich|first2=Ludwig|title=Cough Remedies|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|doi=10.1002/14356007.a08_013|isbn=3527306730}}</ref> | |||
| *] (e.g. ])<ref>{{cite journal|last1=Hropot|first1=Max|last2=Lang|first2=Hans-Jochen|title=Diuretics|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|doi=10.1002/14356007.a09_029|isbn=3527306730}}</ref> | |||
| *]s (e.g. ] and ])<ref name=William /><ref name=Vogel>{{cite book|author1=Furniss, Brian |author2=Hannaford, Antony |author3=Smith, Peter |author4=Tatchell, Austin |name-list-style=amp |title=Vogel's Textbook of Practical Organic Chemistry 5th Ed.|year=1996|publisher=Longman Science & Technical|location=London|isbn=9780582462366|pages=1174–1179|url=https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd}}</ref> & ] | |||
| *] (e.g. ] and ])<ref name=William /><ref>{{cite journal|last1=Bungardt|first1=Edwin|last2=Mutschler|first2=Ernst|title=Spasmolytics|journal=Ullmann's Encyclopedia of Industrial Chemistry|date=2000|doi=10.1002/14356007.a24_515|isbn=3527306730}}</ref> | |||
| *] (e.g. ])<ref name=William /> | |||
| *] (e.g. ], ], and ])<ref name=William /> & ] | |||
| ==Regulation== | |||
| Because benzyl cyanide is a useful ] to numerous ], many countries strictly regulate the compound. | |||
| ===United States=== | |||
| Benzyl cyanide is regulated in the United States as a ]. | |||
| ===China=== | |||
| Benzyl cyanide is regulated in People's Republic of China as a Class III drug precursor since 7 June 2021.<ref>{{cite web|url=http://www.gov.cn/zhengce/content/2021-06/07/content_5615890.htm|title=国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函|publisher=The State Council - The People's Republic of China|language=zh-hans|date=7 June 2021|accessdate=11 October 2021}}</ref> | |||
| ==Safety== | ==Safety== | ||
| Benzyl cyanide, like related benzyl derivatives, is an irritant to the skin and eyes.<ref name=Ullmann/> |
Benzyl cyanide, like related benzyl derivatives, is an irritant to the skin and eyes.<ref name=Ullmann/> | ||
| ==See also== | |||
| *] | |||
| ==References== | ==References== | ||
| {{reflist}} | |||
| <references/> | |||
| ==External links== | ==External links== | ||
| * | * | ||
| {{Authority control}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 17:10, 4 January 2025
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Phenylacetonitrile | |
| Other names
Benzyl cyanide 2-Phenylacetonitrile α-Tolunitrile Benzylnitrile | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.919 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H7N |
| Molar mass | 117.15 g/mol |
| Appearance | Colorless oily liquid |
| Density | 1.015 g/cm |
| Melting point | −24 °C (−11 °F; 249 K) |
| Boiling point | 233 to 234 °C (451 to 453 °F; 506 to 507 K) |
| Magnetic susceptibility (χ) | -76.87·10 cm/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
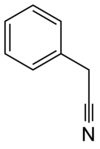
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.
Preparation
Benzyl cyanide can be produced via Kolbe nitrile synthesis between benzyl chloride and sodium cyanide and by oxidative decarboxylation of phenylalanine.
Benzyl cyanides can also be prepared by arylation of silyl-substituted acetonitrile.
Reactions
Benzyl cyanide undergoes many reactions characteristic of nitriles. It can be hydrolyzed to give phenylacetic acid or it can be used in the Pinner reaction to yield phenylacetic acid esters. Hydrogenation gives β-phenethylamine.
The compound contains an "active methylene unit". Bromination occurs gives PhCHBrCN. A variety of base-induced reactions result in the formation of new carbon-carbon bonds.
Uses
Benzyl cyanide is used as a solvent and as a starting material in the synthesis of fungicides (e.g. Fenapanil), fragrances (phenethyl alcohol), antibiotics, and other pharmaceuticals. The partial hydrolysis of BnCN results in 2-phenylacetamide.
Pharmaceuticals
Benzyl cyanide is a useful precursor to numerous pharmaceuticals. Examples include:
- Antiarrhythmics (e.g. disopyramide)
- Antidepressants: E.g. Milnacipran & Lomevactone
- Antihistamines (e.g. levocabastine (para-fluoro), Pheniramine & Azatadine.
- Antitussives (e.g. isoaminile, oxeladin, butethamate, pentapiperide, and pentoxyverine)
- Diuretics (e.g. triamterene)
- Hypnotics (e.g. alonimid and phenobarbital) & Phenglutarimide
- Spasmolytics (e.g. pentapiperide and drofenine)
- Stimulants (e.g. methylphenidate)
- Opioids (e.g. ethoheptazine, pethidine, and phenoperidine) & methadone
Regulation
Because benzyl cyanide is a useful precursor to numerous drugs with recreational use potential, many countries strictly regulate the compound.
United States
Benzyl cyanide is regulated in the United States as a DEA List I chemical.
China
Benzyl cyanide is regulated in People's Republic of China as a Class III drug precursor since 7 June 2021.
Safety
Benzyl cyanide, like related benzyl derivatives, is an irritant to the skin and eyes.
See also
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 16. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- "Toxin Protects Migratory Locusts from Cannibalism". Max Planck Society. 4 May 2023. Retrieved 8 December 2024.
- Adams, Roger; Thal, A. F. (1922). "Benzyl cyanide". Organic Syntheses. 2: 9. doi:10.15227/orgsyn.002.0009.
- Hiegel, Gene; Lewis, Justin; Bae, Jason (2004). "Conversion of α-Amino Acids into Nitriles by Oxidative Decarboxylation with Trichloroisocyanuric Acid". Synthetic Communications. 34 (19): 3449–3453. doi:10.1081/SCC-200030958. S2CID 52208189.
- Wu, Lingyun; Hartwig, John F. (2005). "Mild Palladium-Catalyzed Selective Monoarylation of Nitriles". Journal of the American Chemical Society. 127 (45): 15824–15832. Bibcode:2005JAChS.12715824W. doi:10.1021/ja053027x. PMID 16277525.
- Adams, Roger; Thal, A. F. (1922). "Phenylacetic acid". Organic Syntheses. 2: 59. doi:10.15227/orgsyn.002.0059.
- Adams, Roger; Thal, A. F. (1922). "Ethyl Phenylacetate". Organic Syntheses. 2: 27. doi:10.15227/orgsyn.002.0027.
- Robinson, John C. Jr.; Snyder, H. R. (1943). "β-Phenylethylamine". Organic Syntheses. 23: 71. doi:10.15227/orgsyn.023.0071.
- Robb, C. M.; Schultz, E. M. (1948). "Diphenylacetonitrile". Organic Syntheses. 28: 55. doi:10.15227/orgsyn.028.0055.
- Makosza, M.; Jonczyk, A (1976). "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses. 55: 91. doi:10.15227/orgsyn.055.0091.
- Itoh, Masumi; Hagiwara, Daijiro; Kamiya, Takashi (1988). "New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile". Organic Syntheses. 6: 199. doi:10.15227/orgsyn.059.0095.
- Wawzonek, Stanley; Smolin, Edwin M. (1955). "α-Phenylcinnamonitrile". Organic Syntheses. 3: 715. doi:10.15227/orgsyn.029.0083.
- Bien, Hans-Samuel; Stawitz, Josef; Wunderlich, Klaus (2000). "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry: 29. doi:10.1002/14356007.a02_355. ISBN 3527306730.
- Ackermann, Peter; Margot, Paul; Müller, Franz (2000). "Fungicides, Agricultural". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_085. ISBN 3527306730.
- "PHENYLACETAMIDE". Organic Syntheses. 32: 92. 1952. doi:10.15227/orgsyn.032.0092. ISSN 0078-6209.
- ^ William Andrew Publishing (2008). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Norwich, NY: Elsevier Science. pp. 182, 936, 1362, 1369, 1505, 2036, 2157, 2259, 2554, 2620, 2660, 2670, 2924, 3032, & 3410. ISBN 9780815515265.
- Berkoff, Charles E.; Rivard, Donald E.; Kirkpatrick, David; Ives, Jeffrey L. (1980). "The Reductive Decyanation of Nitriles by Alkali Fusion". Synthetic Communications. 10 (12): 939–945. doi:10.1080/00397918008061855.
- Bub, Oskar; Friedrich, Ludwig (2000). "Cough Remedies". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_013. ISBN 3527306730.
- Hropot, Max; Lang, Hans-Jochen (2000). "Diuretics". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a09_029. ISBN 3527306730.
- Furniss, Brian; Hannaford, Antony; Smith, Peter & Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. pp. 1174–1179. ISBN 9780582462366.
- Bungardt, Edwin; Mutschler, Ernst (2000). "Spasmolytics". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_515. ISBN 3527306730.
- "国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函" (in Simplified Chinese). The State Council - The People's Republic of China. 7 June 2021. Retrieved 11 October 2021.