| Revision as of 08:05, 21 November 2011 editWikitanvirBot (talk | contribs)144,145 editsm r2.7.1) (Robot: Modifying fi:Hupertsiini A← Previous edit | Latest revision as of 00:31, 7 September 2024 edit undoHodgeBrad (talk | contribs)285 editsm Added a line and reference about it being used to help induce lucid dreaming. | ||
| (196 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{chembox | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| | verifiedrevid = 451619690 | |||
| {{Drugbox | |||
| | Name = Huperzine A | |||
| | Verifiedfields = changed | |||
| | ImageFile = Huperzine A.png | |||
| | Watchedfields = changed | |||
| | ImageSize = 200px | |||
| | verifiedrevid = 461740300 | |||
| | ImageName = Huperzine A | |||
| | |
| image = Huperzine A.png | ||
| | |
| width = 200 | ||
| | image2 = HuperzineA3d.png | |||
| | ImageName1 = Huperzine A 3d | |||
| | width2 = 200 | |||
| | IUPACName = (1''R'',9''S'',13''E'')- 1-Amino- 13-ethylidene- 11-methyl- 6-azatricyclo trideca- 2(7),3,10- trien- 5-one | |||
| | IUPAC_name = (1''R'',9''R'',13''E'')-1-Amino-13-ethylidene-11-methyl-6-azatricyclotrideca-2(7),3,10-trien-5-one | |||
| | OtherNames = HupA | |||
| | synonyms = HupA | |||
| | Section1 = {{Chembox Identifiers | |||
| <!--Identifiers--> | |||
| | ATC_prefix = N06 | |||
| | ATC_suffix = | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = DB01928 | | DrugBank = DB01928 | ||
| | PubChem = 854026 | |||
| | SMILES = C\C2=C\\3CC=1NC(=O)\C=C/C=1(N)(C2)C/3=C\C | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 78330 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 395280 | |||
| | ChemSpiderID = 16736021 | | ChemSpiderID = 16736021 | ||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | InChI = 1/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | |||
| | UNII = 0111871I23 | |||
| | InChIKey = ZRJBHWIHUMBLCN-YQEJDHNABN | |||
| | CAS_number_Ref = {{cascite|changed|??}} | |||
| | CAS_number = 102518-79-6 | |||
| <!--Clinical data--> | |||
| | tradename = | |||
| | Drugs.com = | |||
| | legal_AU = | |||
| | legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_UK = | |||
| | legal_US = | |||
| | routes_of_administration = Oral | |||
| <!--Pharmacokinetic data--> | |||
| | bioavailability = | |||
| | protein_bound = | |||
| | metabolism = | |||
| | elimination_half-life = 10-14h<ref>{{cite journal | vauthors = Li YX, Zhang RQ, Li CR, Jiang XH | title = Pharmacokinetics of huperzine A following oral administration to human volunteers | journal = European Journal of Drug Metabolism and Pharmacokinetics | volume = 32 | issue = 4 | pages = 183–187 | date = 2007 | pmid = 18348466 | doi = 10.1007/BF03191002 | s2cid = 2702029 }}</ref> | |||
| | excretion = | |||
| <!--Physical data--> | |||
| | density = | |||
| | melting_point = 217 | |||
| | melting_high = 219 | |||
| | melting_notes = | |||
| | solubility = | |||
| <!--Chemical data--> | |||
| | C=15 | H=18 | N=2 | O=1 | |||
| | smiles = C/C=C/1\2CC3=C(1(CC(=C2)C)N)C=CC(=O)N3 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | | StdInChI = 1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = ZRJBHWIHUMBLCN-YQEJDHNASA-N | | StdInChIKey = ZRJBHWIHUMBLCN-YQEJDHNASA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 102518-79-6 | |||
| | RTECS = | |||
| }} | |||
| | Section2 = {{Chembox Properties | |||
| | Formula = C<sub>15</sub>H<sub>18</sub>N<sub>2</sub>O | |||
| | MolarMass = 242.32 g/mol | |||
| | Appearance = | |||
| | Density = | |||
| | Solubility = | |||
| | MeltingPt = 217–219 °C | |||
| | BoilingPt = | |||
| | pKb = | |||
| }} | |||
| <!-- | |||
| | </nowiki><sub>D</sub>]] | |||
| | -147° (''c'' = 0.36, ]) | |||
| |- | |||
| --> | |||
| | Section7 = {{Chembox Hazards | |||
| | ExternalMSDS = | |||
| | MainHazards = | |||
| | FlashPt = | |||
| | RPhrases = | |||
| | SPhrases = | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | OtherCpds = | |||
| }} | |||
| }} | }} | ||
| '''Huperzine A''' is a naturally-occurring ] ] compound found in the ] '']''<ref name="Zangara2003"/> and in varying quantities in other food ''Huperzia'' species, including ''H. elmeri'', ''H. carinat'', and ''H. aqualupian''.<ref>{{cite journal | vauthors = Lim WH, Goodger JQ, Field AR, Holtum JA, Woodrow IE | title = Huperzine alkaloids from Australasian and southeast Asian Huperzia | journal = Pharmaceutical Biology | volume = 48 | issue = 9 | pages = 1073–1078 | date = September 2010 | pmid = 20731560 | doi = 10.3109/13880209.2010.485619 | doi-access = free }}</ref> Huperzine A has been investigated as a treatment for neurological conditions such as ], but a 2013 ] of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution.<ref name="meta2013">{{cite journal | vauthors = Yang G, Wang Y, Tian J, Liu JP | title = Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials | journal = PLOS ONE | volume = 8 | issue = 9 | pages = e74916 | date = 2013 | pmid = 24086396 | pmc = 3781107 | doi = 10.1371/journal.pone.0074916 | doi-access = free | bibcode = 2013PLoSO...874916Y }}</ref><ref name=cochranemeta /> Huperzine A inhibits the breakdown of the ] ] (ACh) by the enzyme ]. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a ] and marketed as a memory and concentration ]. | |||
| '''Huperzine A''' is a naturally occurring sesquiterpene ] compound found in the plant ] '']''.<ref>{{cite journal|author = Kozikowski, Alan P.; Tueckmantel, Werner|title = Chemistry, Pharmacology, and Clinical Efficacy of the Chinese Nootropic Agent Huperzine A|journal = Accounts of Chemical Research|year = 1999|volume = 32|issue = 8|pages = 641–650|doi = 10.1021/ar9800892}}</ref> | |||
| Huperzine A has also been noted to help induce ].<ref name="Ferris 2009">{{cite web|title=Lucid Dreaming: A Beginner's Guide|url=http://fourhourworkweek.com/2009/09/21/how-to-lucid-dream/|website=The Four Hour Work Week|accessdate=29 December 2016}}</ref> | |||
| Huperzine A is also an ], which has a mechanism of action similar to ], ], and ]. A pro-drug form of Huperzine A (ZT-1) is under development as a treatment for Alzheimer's disease.<ref> Neurobiology of Aging Conference in New Orleans, Nov 2003.</ref> | |||
| In the US, Huperzine A is sold as a dietary supplement for memory support. The botanical has been used in ] for centuries for the treatment of swelling, fever and blood disorders. Clinical trials in China have shown it to be effective in the treatment of Alzheimer's disease<ref name=china>{{cite journal|doi=10.1007/s00702-009-0189-x|title=Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis|year=2009|last1=Wang|first1=Bai-Song|last2=Wang|first2=Hao|last3=Wei|first3=Zhao-hui|last4=Song|first4=Yan-yan|last5=Zhang|first5=Lu|last6=Chen|first6=Hong-Zhuan|journal=Journal of Neural Transmission|volume=116|pages=457|pmid=19221692|issue=4}}</ref> and enhancing memory in students.<ref>{{cite journal|pmid=10678121|year=1999|last1=Sun|first1=QQ|last2=Xu|first2=SS|last3=Pan|first3=JL|last4=Guo|first4=HM|last5=Cao|first5=WQ|title=Huperzine-A capsules enhance memory and learning performance in 34 pairs of matched adolescent students.|volume=20|issue=7|pages=601–3|journal=Zhongguo yao li xue bao = Acta pharmacologica Sinica}}</ref> | |||
| ==Pharmacological effects== | ==Pharmacological effects== | ||
| Huperzine A is extracted from '']''.<ref name="Zangara2003">{{cite journal | vauthors = Zangara A | title = The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease | journal = Pharmacology, Biochemistry, and Behavior | volume = 75 | issue = 3 | pages = 675–686 | date = June 2003 | pmid = 12895686 | doi = 10.1016/S0091-3057(03)00111-4 | s2cid = 36435892 }}</ref> It is a reversible ]<ref>{{cite journal | vauthors = Wang R, Yan H, Tang XC | title = Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine | journal = Acta Pharmacologica Sinica | volume = 27 | issue = 1 | pages = 1–26 | date = January 2006 | pmid = 16364207 | doi = 10.1111/j.1745-7254.2006.00255.x | quote = Huperzine A (HupA), a novel alkaloid isolated from the Chinese herb Huperzia serrata, is a potent, highly specific and reversible inhibitor of acetylcholinesterase (AChE). | doi-access = free }}</ref><ref>{{cite book | url=https://books.google.com/books?id=a5AMBY9ekzcC&q=Huperzine&pg=PA191 | title=Herbs and Nutrients for the Mind: A Guide to Natural Brain Enhancers | publisher=Greenwood Publishing Group | vauthors = Meletis CD, Barke JE | date=2004 | pages=191 | isbn=978-0-275-98394-9}}</ref><ref name="Alzheimer 1996">{{cite journal | vauthors = Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ | title = Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis | journal = Journal of Neural Transmission | volume = 116 | issue = 4 | pages = 457–465 | date = April 2009 | pmid = 19221692 | doi = 10.1007/s00702-009-0189-x | s2cid = 8655284 }}</ref><ref>{{cite journal | vauthors = Tang XC, He XC, Bai DL | title=Huperzine A: A novel acetylcholinesterase inhibitor | journal=Drugs of the Future | date=1999 | volume=24 | issue=6 | pages=647 | doi=10.1358/dof.1999.024.06.545143 }}</ref> and ] antagonist<ref>{{cite journal | vauthors = Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, Nambiar MP | title = -Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats | journal = Chemico-Biological Interactions | volume = 175 | issue = 1–3 | pages = 387–395 | date = September 2008 | pmid = 18588864 | doi = 10.1016/j.cbi.2008.05.023 }}</ref> that crosses the ].<ref>{{cite journal | vauthors = Patocka J | title = Huperzine A--an interesting anticholinesterase compound from the Chinese herbal medicine | journal = Acta Medica | volume = 41 | issue = 4 | pages = 155–157 | date = 1998 | pmid = 9951045 | doi = 10.14712/18059694.2019.181 | doi-access = free }}</ref> ] is an enzyme that catalyzes the breakdown of the neurotransmitter ACh and other choline esters that function as neurotransmitters. The structure of the complex of huperzine A with acetylcholinesterase has been determined by ] (PDB code: ; ).<ref>{{cite journal | vauthors = Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL | title = Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A | journal = Nature Structural Biology | volume = 4 | issue = 1 | pages = 57–63 | date = January 1997 | pmid = 8989325 | doi = 10.1038/nsb0197-57 | s2cid = 236518 }}</ref> | |||
| Huperzine A is an acetylcholinesterase inhibititor<ref name="Alzheimer 1996">{{cite journal|pmid=19221692|year=2009|last1=Wang|first1=BS|last2=Wang|first2=H|last3=Wei|first3=ZH|last4=Song|first4=YY|last5=Zhang|first5=L|last6=Chen|first6=HZ|title=Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis.|volume=116|issue=4|pages=457–65|doi=10.1007/s00702-009-0189-x|journal=Journal of neural transmission (Vienna, Austria : 1996)}}</ref> and ] antagonist,<ref>{{cite journal|pmid=18588864|year=2008|last1=Coleman|first1=BR|last2=Ratcliffe|first2=RH|last3=Oguntayo|first3=SA|last4=Shi|first4=X|last5=Doctor|first5=BP|last6=Gordon|first6=RK|last7=Nambiar|first7=MP|title=+-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats.|volume=175|issue=1-3|pages=387–95|doi=10.1016/j.cbi.2008.05.023|journal=Chemico-biological interactions}}</ref>. | |||
| Huperzine A has been investigated as a possible treatment for diseases characterized by ] such as ],<ref name="Zangara2003" /><ref name="r1">{{cite journal | vauthors = Bai DL, Tang XC, He XC | title = Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease | journal = Current Medicinal Chemistry | volume = 7 | issue = 3 | pages = 355–374 | date = March 2000 | pmid = 10637369 | doi = 10.2174/0929867003375281 }}</ref> and there is some evidence from small-scale studies that it can benefit cognitive functioning, global clinical status, and ability to engage in ] (ADLs) among individuals with the disease. In a 2016 ],<ref>{{cite journal | vauthors = Laver K, Dyer S, Whitehead C, Clemson L, Crotty M | title = Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews | journal = BMJ Open | volume = 6 | issue = 4 | pages = e010767 | date = April 2016 | pmid = 27121704 | pmc = 4854009 | doi = 10.1136/bmjopen-2015-010767 }}</ref> huperzine A was associated with a ] of 1.48 (], 0.95-2.02) compared to placebo on measures of ADL among people with dementia, but the evidence was very low-quality and uncertain. In a 2022 umbrella review,<ref>{{cite journal | vauthors = Fan F, Liu H, Shi X, Ai Y, Liu Q, Cheng Y | title = The Efficacy and Safety of Alzheimer's Disease Therapies: An Updated Umbrella Review | journal = Journal of Alzheimer's Disease | volume = 85 | issue = 3 | pages = 1195–1204 | date = 2022-02-01 | pmid = 34924395 | doi = 10.3233/JAD-215423 | s2cid = 245311001 }}</ref> huperzine A was associated with broad benefits to dementia patients' cognitive functioning, but the degree of ] in measurements and outcomes of the reviewed studies indicated ] toward huperzine A benefit. | |||
| Huperzine A has also attracted the attention of US medical science. It is currently being investigated as a possible treatment for diseases characterized by neurodegeneration – particularly ].<ref>{{cite journal|author = Zangara, A|title = The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease|journal = Pharmacol Biochem Behav.|year = 2003|volume = 75|issue = 3|pages = 675–86|doi = 10.1016/S0091-3057(03)00111-4|pmid = 12895686}}</ref><ref name=r1>{{cite journal|author = Bai, D. L.; Tang, X. C.; He, X. C.|title = Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease|pmid=10637369|journal = Current Medicinal Chemistry|year = 2000|volume = 7 |issue= 3|pages = 355–374}}</ref> It has been found to be an ] of the enzyme ].<ref>{{cite journal|author = Tang, X. C.; He, X. C.; Bai, D. L.|title = Huperzine A: a novel acetylcholinesterase inhibitor|journal = Drugs of the Future|year = 1999|volume = 24|issue = 6|pages = 647–663|doi = 10.1358/dof.1999.024.06.545143}}</ref> The structure of the complex of huperzine A with acetylcholinesterase has been determined<ref>{{cite journal|pmid= 8989325|year=1997|last1= Raves |first1=ML|last2=Harel|first2=M|last3=Pang|first3=Y-P|last4=Silman|first4=I|last5=Kozikowski|first5=AP|last6=Sussman|first6=JL|title=3D structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A.|volume=4|issue=1|pages=57–63|journal=Nature Struct Biol}}</ref>. by ] (PDB code: ; ).This is the same ] of pharmaceutical drugs such as ] and ] used to treat Alzheimer's disease. Huperzine A is also a ] ] which protects the brain against glutamate induced damage, and it appears to increase ] levels in rats.<ref>{{cite journal|author = Tang, L., Wang, R., Tang, X.|title = Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells|journal = Acta Pharmacologica Sinica|year = 2005|volume = 26|pages = 673–678|doi = 10.1111/j.1745-7254.2005.00130.x|pmid = 15916732|issue = 6}}</ref> | |||
| ==Adverse effects== | |||
| Clinical trials in China have shown that huperzine A is comparably effective to similar drugs on the market, and may even be a bit safer in terms of side effects.<ref name=china/> The ] has completed<ref name=r1/> a Phase II clinical trial<ref>, Alzherimer Research Forum</ref> to evaluate the safety and efficacy of huperzine A in the treatment of Alzheimer's disease in a ] of its effect on cognitive function.The study concluded that huperzine A 200 mug BID has no demonstrable cognitive effect in patients with mild to moderate AD.<ref>Rafii M.S., Walsh S., Little J.T., Behan K., Reynolds B., Ward C., Jin S., Thomas R., Aisen P.S.,"A phase II trial of huperzine A in mild to moderate Alzheimer disease". ''Neurology''. 76 (16) (pp 1389-1394), 2011.</ref> | |||
| Huperzine A may present with mild ] side effects such as nausea, vomiting, and diarrhea.<ref name="cochranemeta">{{cite journal | vauthors = Li J, Wu HM, Zhou RL, Liu GJ, Dong BR | veditors = Wu HM | title = Huperzine A for Alzheimer's disease | journal = The Cochrane Database of Systematic Reviews | volume = CD005592 | issue = 2 | pages = CD005592 | date = April 2008 | pmid = 18425924 | doi = 10.1002/14651858.CD005592.pub2 }}</ref> Slight muscle twitching and slurred speech might also occur, as well as ] and sweating. The use of huperzine A during pregnancy and lactation is not recommended due to the lack of sufficient safety data.<ref name="Natural Standard">{{cite web|title=Huperzine A|url=https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=764#safety|website=Natural Standard: The Authority on Integrative Medicine |publisher=Natural Standard|access-date=29 October 2014}}</ref> | |||
| ==Drug interactions== | |||
| Possible side effects include breathing problems, tightness in the throat or chest, chest pain, skin hives, rash, itchy or swollen skin, upset stomach, ], vomiting, ] and ]. Most adverse events were cholinergic in nature and no serious adverse events occurred. Huperzine A is a well-tolerated drug.<ref name="Alzheimer 1996"/> | |||
| Huperzine A may have ] if taken with drugs causing ], such as ]s,<ref name="pepping">{{cite journal | vauthors = Pepping J | title = Huperzine A | journal = American Journal of Health-System Pharmacy | volume = 57 | issue = 6 | pages = 530, 533-530, 534 | date = March 2000 | pmid = 10754762 | doi = 10.1093/ajhp/57.6.530 | doi-access = free }}</ref> which may decrease heart rate. Theoretically, there may be possible additive cholinergic effects if huperzine A is taken with other ]s or ] agents.<ref>{{cite journal | vauthors = Skolnick AA | title = Old Chinese herbal medicine used for fever yields possible new Alzheimer disease therapy | journal = JAMA | volume = 277 | issue = 10 | pages = 776 | date = March 1997 | pmid = 9052690 | doi = 10.1001/jama.1997.03540340010004 }}</ref> | |||
| == |
==Safety== | ||
| Huperzine A, in spite of the possible cholinergic side effects, seems to have a wide margin of safety. ] studies show huperzine A to be non-toxic even when administered at 50-100 times the human therapeutic dose. The extract is active for 6 hours at a dose of 2 μg/kg with no remarkable side effects.<ref name="Safety">{{cite journal | vauthors = Lallement G, Baille V, Baubichon D, Carpentier P, Collombet JM, Filliat P, Foquin A, Four E, Masqueliez C, Testylier G, Tonduli L, Dorandeu F | title = Review of the value of huperzine as pretreatment of organophosphate poisoning | journal = Neurotoxicology | volume = 23 | issue = 1 | pages = 1–5 | date = May 2002 | pmid = 12164543 | doi = 10.1016/S0161-813X(02)00015-3 }}</ref> | |||
| *] and in ] | |||
| ==Other possible uses== | |||
| ==References== | |||
| Huperzine A might be useful in the treatment of ] by preventing damage to the ] caused by such agents. | |||
| {{reflist|2}} | |||
| <ref name="Poisoning">{{cite web|title=Review of the Value of Huperzine as Pretreatment of Organophosphate Poisoning|url=https://nutritionreview.org/2013/04/huperzinea/|website=Nutrition Review|date=22 April 2013}}</ref> | |||
| <ref name="Poisoning2">{{cite journal | vauthors = Liu L, Sun JX | title = | journal = Wei Sheng Yan Jiu = Journal of Hygiene Research | volume = 34 | issue = 2 | pages = 224–226 | date = March 2005 | pmid = 15952670 | url = https://europepmc.org/article/med/15952670 }}</ref> | |||
| ==Synthesis== | |||
| {{Cholinergics}} | |||
| Two scalable and efficient ] of huperzine A have been reported.<ref>{{cite journal | vauthors = un MK, Wüstmann DJ, Herzon SB | title=A robust and scalable synthesis of the potent neuroprotective agent (−)-huperzine A | journal=Chemical Science | date=2011 | volume=2 | issue=11 | pages=2251–2253 | doi=10.1039/C1SC00455G | s2cid=98224866 }}</ref><ref>{{cite journal | vauthors = Tudhope SR, Bellamy JA, Ball A, Rajasekar D, Azadi-Ardakani M, Meera HS, Gnanadeepam JM, Saiganesh R, Gibson F, He L, Behrens CH | title=Development of a Large-Scale Synthetic Route to Manufacture (−)-Huperzine A | journal=Organic Process Research & Development | date=2012 | volume=16 | issue=4 | pages=635–642 | doi=10.1021/op200360b }}</ref> | |||
| == References == | |||
| {{Reflist|2}} | |||
| == External links == | |||
| * in ] | |||
| {{Antidementia}} | |||
| {{Acetylcholine metabolism and transport modulators}} | |||
| {{Ionotropic glutamate receptor modulators}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:31, 7 September 2024
Chemical compoundPharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Other names | HupA |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 10-14h |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.132.430 |
| Chemical and physical data | |
| Formula | C15H18N2O |
| Molar mass | 242.322 g·mol |
| 3D model (JSmol) | |
| Melting point | 217 to 219 °C (423 to 426 °F) |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
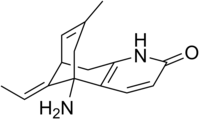
Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
Huperzine A has also been noted to help induce lucid dreaming.
Pharmacological effects
Huperzine A is extracted from Huperzia serrata. It is a reversible acetylcholinesterase inhibitor and NMDA receptor antagonist that crosses the blood–brain barrier. Acetylcholinesterase is an enzyme that catalyzes the breakdown of the neurotransmitter ACh and other choline esters that function as neurotransmitters. The structure of the complex of huperzine A with acetylcholinesterase has been determined by X-ray crystallography (PDB code: 1VOT; see the 3D structure).
Huperzine A has been investigated as a possible treatment for diseases characterized by neurodegeneration such as Alzheimer's disease, and there is some evidence from small-scale studies that it can benefit cognitive functioning, global clinical status, and ability to engage in activities of daily living (ADLs) among individuals with the disease. In a 2016 systematic review of systematic reviews, huperzine A was associated with a standardized mean difference of 1.48 (95% CI, 0.95-2.02) compared to placebo on measures of ADL among people with dementia, but the evidence was very low-quality and uncertain. In a 2022 umbrella review, huperzine A was associated with broad benefits to dementia patients' cognitive functioning, but the degree of heterogeneity in measurements and outcomes of the reviewed studies indicated publication bias toward huperzine A benefit.
Adverse effects
Huperzine A may present with mild cholinergic side effects such as nausea, vomiting, and diarrhea. Slight muscle twitching and slurred speech might also occur, as well as excessive saliva excretion and sweating. The use of huperzine A during pregnancy and lactation is not recommended due to the lack of sufficient safety data.
Drug interactions
Huperzine A may have additive effects if taken with drugs causing bradycardia, such as beta-blockers, which may decrease heart rate. Theoretically, there may be possible additive cholinergic effects if huperzine A is taken with other acetylcholinesterase inhibitors or cholinergic agents.
Safety
Huperzine A, in spite of the possible cholinergic side effects, seems to have a wide margin of safety. Toxicology studies show huperzine A to be non-toxic even when administered at 50-100 times the human therapeutic dose. The extract is active for 6 hours at a dose of 2 μg/kg with no remarkable side effects.
Other possible uses
Huperzine A might be useful in the treatment of organophosphate nerve agent poisoning by preventing damage to the central nervous system caused by such agents.
Synthesis
Two scalable and efficient total syntheses of huperzine A have been reported.
References
- Li YX, Zhang RQ, Li CR, Jiang XH (2007). "Pharmacokinetics of huperzine A following oral administration to human volunteers". European Journal of Drug Metabolism and Pharmacokinetics. 32 (4): 183–187. doi:10.1007/BF03191002. PMID 18348466. S2CID 2702029.
- ^ Zangara A (June 2003). "The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease". Pharmacology, Biochemistry, and Behavior. 75 (3): 675–686. doi:10.1016/S0091-3057(03)00111-4. PMID 12895686. S2CID 36435892.
- Lim WH, Goodger JQ, Field AR, Holtum JA, Woodrow IE (September 2010). "Huperzine alkaloids from Australasian and southeast Asian Huperzia". Pharmaceutical Biology. 48 (9): 1073–1078. doi:10.3109/13880209.2010.485619. PMID 20731560.
- Yang G, Wang Y, Tian J, Liu JP (2013). "Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials". PLOS ONE. 8 (9): e74916. Bibcode:2013PLoSO...874916Y. doi:10.1371/journal.pone.0074916. PMC 3781107. PMID 24086396.
- ^ Li J, Wu HM, Zhou RL, Liu GJ, Dong BR (April 2008). Wu HM (ed.). "Huperzine A for Alzheimer's disease". The Cochrane Database of Systematic Reviews. CD005592 (2): CD005592. doi:10.1002/14651858.CD005592.pub2. PMID 18425924.
- "Lucid Dreaming: A Beginner's Guide". The Four Hour Work Week. Retrieved 29 December 2016.
- Wang R, Yan H, Tang XC (January 2006). "Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine". Acta Pharmacologica Sinica. 27 (1): 1–26. doi:10.1111/j.1745-7254.2006.00255.x. PMID 16364207.
Huperzine A (HupA), a novel alkaloid isolated from the Chinese herb Huperzia serrata, is a potent, highly specific and reversible inhibitor of acetylcholinesterase (AChE).
- Meletis CD, Barke JE (2004). Herbs and Nutrients for the Mind: A Guide to Natural Brain Enhancers. Greenwood Publishing Group. p. 191. ISBN 978-0-275-98394-9.
- Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ (April 2009). "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of Neural Transmission. 116 (4): 457–465. doi:10.1007/s00702-009-0189-x. PMID 19221692. S2CID 8655284.
- Tang XC, He XC, Bai DL (1999). "Huperzine A: A novel acetylcholinesterase inhibitor". Drugs of the Future. 24 (6): 647. doi:10.1358/dof.1999.024.06.545143.
- Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, et al. (September 2008). "-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats". Chemico-Biological Interactions. 175 (1–3): 387–395. doi:10.1016/j.cbi.2008.05.023. PMID 18588864.
- Patocka J (1998). "Huperzine A--an interesting anticholinesterase compound from the Chinese herbal medicine". Acta Medica. 41 (4): 155–157. doi:10.14712/18059694.2019.181. PMID 9951045.
- Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL (January 1997). "Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A". Nature Structural Biology. 4 (1): 57–63. doi:10.1038/nsb0197-57. PMID 8989325. S2CID 236518.
- Bai DL, Tang XC, He XC (March 2000). "Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease". Current Medicinal Chemistry. 7 (3): 355–374. doi:10.2174/0929867003375281. PMID 10637369.
- Laver K, Dyer S, Whitehead C, Clemson L, Crotty M (April 2016). "Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews". BMJ Open. 6 (4): e010767. doi:10.1136/bmjopen-2015-010767. PMC 4854009. PMID 27121704.
- Fan F, Liu H, Shi X, Ai Y, Liu Q, Cheng Y (2022-02-01). "The Efficacy and Safety of Alzheimer's Disease Therapies: An Updated Umbrella Review". Journal of Alzheimer's Disease. 85 (3): 1195–1204. doi:10.3233/JAD-215423. PMID 34924395. S2CID 245311001.
- "Huperzine A". Natural Standard: The Authority on Integrative Medicine. Natural Standard. Retrieved 29 October 2014.
- Pepping J (March 2000). "Huperzine A". American Journal of Health-System Pharmacy. 57 (6): 530, 533–530, 534. doi:10.1093/ajhp/57.6.530. PMID 10754762.
- Skolnick AA (March 1997). "Old Chinese herbal medicine used for fever yields possible new Alzheimer disease therapy". JAMA. 277 (10): 776. doi:10.1001/jama.1997.03540340010004. PMID 9052690.
- Lallement G, Baille V, Baubichon D, Carpentier P, Collombet JM, Filliat P, et al. (May 2002). "Review of the value of huperzine as pretreatment of organophosphate poisoning". Neurotoxicology. 23 (1): 1–5. doi:10.1016/S0161-813X(02)00015-3. PMID 12164543.
- "Review of the Value of Huperzine as Pretreatment of Organophosphate Poisoning". Nutrition Review. 22 April 2013.
- Liu L, Sun JX (March 2005). "[Advances on study of organophosphate poisoning prevented by Huperzine A]". Wei Sheng Yan Jiu = Journal of Hygiene Research. 34 (2): 224–226. PMID 15952670.
- un MK, Wüstmann DJ, Herzon SB (2011). "A robust and scalable synthesis of the potent neuroprotective agent (−)-huperzine A". Chemical Science. 2 (11): 2251–2253. doi:10.1039/C1SC00455G. S2CID 98224866.
- Tudhope SR, Bellamy JA, Ball A, Rajasekar D, Azadi-Ardakani M, Meera HS, et al. (2012). "Development of a Large-Scale Synthetic Route to Manufacture (−)-Huperzine A". Organic Process Research & Development. 16 (4): 635–642. doi:10.1021/op200360b.
External links
| Psychoanaleptics: Anti-dementia agents (ATC code N06D and others) | |
|---|---|
| AChE inhibitor medications | |
| Other medications | |
| Experimental BACE inhibitors | |
| Ionotropic glutamate receptor modulators | |
|---|---|
| AMPARTooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
|
| KARTooltip Kainate receptor |
|
| NMDARTooltip N-Methyl-D-aspartate receptor |
|