| Revision as of 06:17, 27 November 2011 editVolkovBot (talk | contribs)447,718 editsm r2.5.1) (Robot: Modifying de:9,10-Bis(phenylethinyl)anthracen← Previous edit | Latest revision as of 23:08, 7 June 2023 edit undoReba16 (talk | contribs)Extended confirmed users8,067 editsmNo edit summaryTag: Manual revert | ||
| (22 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 477227525 | ||
| | ImageFile = 9,10-bis(phenylethynyl)anthracene.svg | | ImageFile = 9,10-bis(phenylethynyl)anthracene.svg | ||
| | ImageSize = 250px | | ImageSize = 250px | ||
| Line 7: | Line 9: | ||
| | ImageSize1 = 250px | | ImageSize1 = 250px | ||
| | ImageName1 = Ball-and-stick model | | ImageName1 = Ball-and-stick model | ||
| | |
| PIN = 9,10-Bis(phenylethynyl)anthracene | ||
| | OtherNames = 9,10-Bis(phenylethynyl)anthracene, BPEA | | OtherNames = 9,10-Bis(2-phenylethynyl)anthracene, BPEA | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | |
| InChI = 1/C30H18/c1-3-11-23(12-4-1)19-21-29-25-15-7-9-17-27(25)30(28-18-10-8-16-26(28)29)22-20-24-13-5-2-6-14-24/h1-18H | ||
| | InChIKey = ZHBOFZNNPZNWGB-UHFFFAOYAU | | InChIKey = ZHBOFZNNPZNWGB-UHFFFAOYAU | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 16: | Line 18: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = ZHBOFZNNPZNWGB-UHFFFAOYSA-N | | StdInChIKey = ZHBOFZNNPZNWGB-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|changed|??}} | |||
| | CASNo = 10075-85-1 | | CASNo = 10075-85-1 | ||
| | |
| Beilstein = 1891432 | ||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| ⚫ | | |
||
| | UNII = FC8JDB70DQ | |||
| ⚫ | | |
||
| | EINECS = 233-210-8 | |||
| ⚫ | | PubChem = 82338 | ||
| ⚫ | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 51675 | | ChEBI = 51675 | ||
| | SMILES = C(#Cc1ccccc1)c4c2ccccc2c(C#Cc3ccccc3)c5ccccc45 | | SMILES = C(#Cc1ccccc1)c4c2ccccc2c(C#Cc3ccccc3)c5ccccc45 | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 74309 | | ChemSpiderID = 74309 | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = C<sub>30</sub>H<sub>18</sub> | ||
| | |
| MolarMass = 378.473 g/mol | ||
| | |
| Appearance = Orange needle crystals | ||
| | |
| Density = | ||
| | |
| MeltingPtC = 252 to 258 | ||
| | |
| BoilingPt = | ||
| | |
| Solubility = | ||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | |
| MainHazards = Irritant ('''Xi''') | ||
| | |
| FlashPtC= | ||
| | |
| AutoignitionPtC = | ||
| | |
| GHSPictograms = {{GHS07}} | ||
| | GHSSignalWord = Warning | |||
| | SPhrases = {{S26}} {{S36}} {{S37/39}} {{S45}} {{S28}}A | |||
| | HPhrases = {{H-phrases|315|319|335}} | |||
| | PPhrases = {{P-phrases|261|264|271|280|302+352|304+340|305+351+338|312|321|332+313|337+313|362|403+233|405|501}} | |||
| }} | }} | ||
| }} | }} | ||
| Line 45: | Line 53: | ||
| It is used in ]s as a ] producing ghostly green light. It is also used as a ] for ]s in ]. | It is used in ]s as a ] producing ghostly green light. It is also used as a ] for ]s in ]. | ||
| ⚫ | ] | ||
| The emission wavelength can be lowered by substituting the ] core by ]s or ]s. 2-ethyl and 1,2-dimethyl substituted BPEAs are also in use. | The emission wavelength can be lowered by substituting the ] core by ]s or ]s. 2-ethyl and 1,2-dimethyl substituted BPEAs are also in use. | ||
| Line 58: | Line 69: | ||
| == External links == | == External links == | ||
| * | * | ||
| * | * | ||
| * | * | ||
| Line 65: | Line 76: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ⚫ | ] | ||
| ] | |||
Latest revision as of 23:08, 7 June 2023

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 9,10-Bis(phenylethynyl)anthracene | |
| Other names 9,10-Bis(2-phenylethynyl)anthracene, BPEA | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1891432 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.178 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C30H18 |
| Molar mass | 378.473 g/mol |
| Appearance | Orange needle crystals |
| Melting point | 252 to 258 °C (486 to 496 °F; 525 to 531 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant (Xi) |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
9,10-Bis(phenylethynyl)anthracene (BPEA) is an aromatic hydrocarbon with the chemical formula is C30H18. It displays strong fluorescence and is used as a chemiluminescent fluorophore with high quantum efficiency.
It is used in lightsticks as a fluorophor producing ghostly green light. It is also used as a dopant for organic semiconductors in OLEDs.
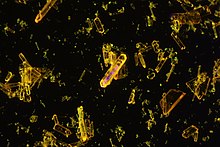
The emission wavelength can be lowered by substituting the anthracene core by halogens or alkyls. 2-ethyl and 1,2-dimethyl substituted BPEAs are also in use.
- 1-chloro-9,10-bis(phenylethynyl)anthracene emits yellow-green light, used in 30-minute high-intensity Cyalume sticks
- 2-chloro-9,10-bis(phenylethynyl)anthracene emits green light, used in 12-hour low-intensity Cyalume sticks
See also
External links
- Absorption and emission spectra
- National Pollutant Inventory - Polycyclic Aromatic Hydrocarbon Fact Sheet
- Novel red-emitting BPEAs