| Revision as of 12:41, 19 December 2011 edit129.67.119.242 (talk)No edit summary← Previous edit | Latest revision as of 22:34, 12 August 2023 edit undoTeaktl17 (talk | contribs)Extended confirmed users18,246 edits →See also: alt. names | ||
| (29 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{lowercasetitle}} | |||
| {{DISPLAYTITLE:''alpha''-Ketobutyric acid}} | |||
| {{ |
{{Chembox | ||
| | verifiedrevid = |
| verifiedrevid = 477319185 | ||
| |Name=α-Ketobutyric acid | | Name = α-Ketobutyric acid | ||
| |ImageFile=Alpha-ketobutyric acid.svg | | ImageFile = Alpha-ketobutyric acid.svg | ||
| |ImageSize= | | ImageSize = | ||
| |ImageFile1= |
| ImageFile1 = 2-Oxobutanoic acid 3D ball.png | ||
| | ImageSize1 = | |||
| |ImageSize= | |||
| | |
| PIN = 2-Oxobutanoic acid | ||
| |OtherNames= | | OtherNames = | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C00109 | | KEGG = C00109 | ||
| | InChI = 1/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7) | | InChI = 1/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7) | ||
| Line 16: | Line 16: | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 171246 | | ChEMBL = 171246 | ||
| | EINECS = 209-986-9 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7) | | StdInChI = 1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7) | ||
| Line 21: | Line 22: | ||
| | StdInChIKey = TYEYBOSBBBHJIV-UHFFFAOYSA-N | | StdInChIKey = TYEYBOSBBBHJIV-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo=600-18-0 | | CASNo = 600-18-0 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = B92RB6HY1A | |||
| ⚫ | | |
||
| ⚫ | | PubChem = 58 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 57 | | ChemSpiderID = 57 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 30831 | | ChEBI = 30831 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = DB04553 | | DrugBank = DB04553 | ||
| | SMILES = O=C(C(=O)O)CC | | SMILES = O=C(C(=O)O)CC | ||
| | |
| MeSHName = Alpha-ketobutyric+acid | ||
| }} | |||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>4</sub>H<sub>6</sub>O<sub>3</sub> | ||
| | |
| MolarMass = 102.089 g/mol | ||
| | |
| Appearance = colorless solid | ||
| | |
| Density = | ||
| | MeltingPtC = 33 | |||
| | MeltingPt= | |||
| | |
| BoilingPt = | ||
| | |
| Solubility = | ||
| }} | |||
| |Section3= |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| }} | |||
| }} | }} | ||
| ⚫ | '''α-Ketobutyric acid''' is an ] with the formula CH<sub>3</sub>CH<sub>2</sub>C(O)CO<sub>2</sub>H. It is a colorless solid that melts just above room temperature. Its conjugate base '''α-ketobutyrate''' is the predominant form found in nature (near neutral pH). It results from the lysis of ]. It is also one of the degradation products of ], produced by the catabolism of the amino acid by ]. It is also produced by the degradation of ] and the metabolism of ]. | ||
| '''α-Ketobutyric acid''' is a product of the lysis of ]. | |||
| ⚫ | α-Ketobutyrate is transported into the ], where it is converted to ] by ]. Further mitochondrial reactions produce ]. This is first through the enzyme mitochondria ] with ] as a ] to produce (''S'')-]. This is subsequently converted to (''R'')-methylmalonyl-CoA by mitochondrial ]. Finally, mitochondrial ] with cofactor ] produces succinyl-CoA which enters the ].<ref>http://smpdb.ca/ {{nonspecific|date=August 2022}}</ref> | ||
| ⚫ | It is also one of the degradation products of ], produced by the catabolism of the amino acid by ]. It is also produced by the degradation of ] and the metabolism of ]. | ||
| ⚫ | == Conversion in sotolon in French ''vin jaune'' == | ||
| ⚫ | |||
| ⚫ | '']'' is marked by the formation of ] from alpha-ketobutyric acid.<ref>{{ cite journal | vauthors = Pham TT, Guichard E, Schlich P, Charpentier C | title = Optimal Conditions for the Formation of Sotolon from α-Ketobutyric Acid in the French 'Vin Jaune' | journal = Journal of Agricultural and Food Chemistry | year = 1995 | volume = 43 | issue = 10 | pages = 2616–2619 | doi = 10.1021/jf00058a012 }}</ref><ref>{{ cite journal | title = Quantitative Determination of Sotolon in Wines by High-Performance Liquid Chromatography | vauthors = Guichard E, Pham TT, Etievant P | journal = Chromatographia | volume = 37 | issue = 9–10 | pages = 539–542 | doi = 10.1007/BF02275793 | year = 1993 | s2cid = 95494741 }}</ref> | ||
| ⚫ | == Conversion in sotolon in French |
||
| ⚫ | ] is marked by the formation of ] from alpha-ketobutyric acid.<ref>Optimal Conditions for the Formation of Sotolon from |
||
| == See also == | == See also == | ||
| * ] | * ] | ||
| * ] (homoalanine) | |||
| * ] (α-hydroxybutyric acid) | |||
| * Other ]s | |||
| ** ] (acetoacetic acid) | |||
| ** ] (succinic semialdehyde) | |||
| ==References== | ==References== | ||
| Line 66: | Line 72: | ||
| {{DEFAULTSORT:Ketobutyric acid, alpha-}} | {{DEFAULTSORT:Ketobutyric acid, alpha-}} | ||
| ] | ] | ||
| {{biochem-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:34, 12 August 2023
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Oxobutanoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.080 |
| EC Number |
|
| KEGG | |
| MeSH | Alpha-ketobutyric+acid |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O3 |
| Molar mass | 102.089 g/mol |
| Appearance | colorless solid |
| Melting point | 33 °C (91 °F; 306 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
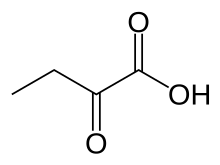
α-Ketobutyric acid is an organic compound with the formula CH3CH2C(O)CO2H. It is a colorless solid that melts just above room temperature. Its conjugate base α-ketobutyrate is the predominant form found in nature (near neutral pH). It results from the lysis of cystathionine. It is also one of the degradation products of threonine, produced by the catabolism of the amino acid by threonine dehydratase. It is also produced by the degradation of homocysteine and the metabolism of methionine.
α-Ketobutyrate is transported into the mitochondrial matrix, where it is converted to propionyl-CoA by branched-chain alpha-keto acid dehydrogenase complex. Further mitochondrial reactions produce succinyl-CoA. This is first through the enzyme mitochondria propionyl-CoA carboxylase with biotin as a cofactor to produce (S)-methylmalonyl-CoA. This is subsequently converted to (R)-methylmalonyl-CoA by mitochondrial methylmalonyl-CoA epimerase. Finally, mitochondrial methylmalonyl-CoA mutase with cofactor adenosylcobalamin produces succinyl-CoA which enters the citric acid cycle.
Conversion in sotolon in French vin jaune
Vin jaune is marked by the formation of sotolon from alpha-ketobutyric acid.
See also
- Butyric acid
- α-Aminobutyric acid (homoalanine)
- 2-Hydroxybutyric acid (α-hydroxybutyric acid)
- Other oxobutanoic acids
- 3-Oxobutanoic acid (acetoacetic acid)
- 4-Oxobutanoic acid (succinic semialdehyde)
References
- http://smpdb.ca/
- Pham TT, Guichard E, Schlich P, Charpentier C (1995). "Optimal Conditions for the Formation of Sotolon from α-Ketobutyric Acid in the French 'Vin Jaune'". Journal of Agricultural and Food Chemistry. 43 (10): 2616–2619. doi:10.1021/jf00058a012.
- Guichard E, Pham TT, Etievant P (1993). "Quantitative Determination of Sotolon in Wines by High-Performance Liquid Chromatography". Chromatographia. 37 (9–10): 539–542. doi:10.1007/BF02275793. S2CID 95494741.