| Revision as of 17:37, 25 January 2012 editR'n'B (talk | contribs)Administrators421,288 editsm Fix links to disambiguation page Nitro← Previous edit | Latest revision as of 17:32, 17 November 2024 edit undoDavemck (talk | contribs)Extended confirmed users120,104 editsm Clean up duplicate template arguments using findargdups; fix ref error | ||
| (59 intermediate revisions by 30 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 477214862 | ||
| ⚫ | | ImageFileL1 = 2-nitrobenzaldehyde.svg | ||
| | Reference=<ref></ref><ref></ref> | |||
| | ImageSizeL1 = 125 | |||
| ⚫ | | |
||
| | ImageAltL1 = Skeletal formula of 2-nitrobenzaldehyde | |||
| | ImageSize = 130px | |||
| | |
| ImageFileR1 = 2-Nitrobenzaldehyde-3D-balls.png | ||
| | ImageSizeR1 = 135 | |||
| ⚫ | | OtherNames = Nitrobenzaldehyde |
||
| | ImageAltR1 = Ball-and-stick model of the 2-nitrobenzaldehyde molecule | |||
| ⚫ | | |
||
| | PIN = 2-Nitrobenzaldehyde | |||
| ⚫ | | |
||
| ⚫ | | OtherNames = Nitrobenzaldehyde<br />''ortho''-Nitrobenzaldehyde<br />''o''-Nitrobenzaldehyde | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | CASNo = 552-89-6 | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | ChEBI = 66927 | |||
| ⚫ | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ⚫ | | ChEMBL = 166559 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 10630 | ||
| | EC_number = 209-025-3 | |||
| ⚫ | | PubChem = 11101 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 48B18Q9B8E | |||
| | InChI = 1/C7H5NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H | | InChI = 1/C7H5NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H | ||
| ⚫ | | SMILES = O=()c1ccccc1C=O | ||
| | InChIKey = CMWKITSNTDAEDT-UHFFFAOYAD | | InChIKey = CMWKITSNTDAEDT-UHFFFAOYAD | ||
| ⚫ | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ⚫ | | ChEMBL = 166559 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C7H5NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H | | StdInChI = 1S/C7H5NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = CMWKITSNTDAEDT-UHFFFAOYSA-N | | StdInChIKey = CMWKITSNTDAEDT-UHFFFAOYSA-N | ||
| ⚫ | | SMILES = O=()c1ccccc1C=O | ||
| ⚫ | | CASNo = 552-89-6 | ||
| ⚫ | | |
||
| ⚫ | | |
||
| ⚫ | | ChemSpiderID = 10630 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>7</sub>H<sub>5</sub>NO<sub>3</sub> | ||
| | |
| MolarMass = 151.12 g/mol | ||
| | |
| Appearance = Pale yellow crystalline powder | ||
| | |
| Density = | ||
| | |
| MeltingPtC = 43 | ||
| | |
| BoilingPtC = 152 | ||
| | |
| Solubility = Insoluble | ||
| | MagSus = -68.23·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | |
| MainHazards = Harmful, Potentially mutagenic | ||
| | GHS_ref=<ref>{{cite web |title=2-Nitrobenzaldehyde |url=https://pubchem.ncbi.nlm.nih.gov/compound/11101#section=Safety-and-Hazards |website=pubchem.ncbi.nlm.nih.gov |language=en}}</ref> | |||
| | RPhrases = {{R36}} {{R37}} {{R38}} {{R41}} | |||
| | |
| GHSPictograms = {{GHS07}} | ||
| | GHSSignalWord = Warning | |||
| ⚫ | | |
||
| | HPhrases = {{H-phrases|302|315|319|335|412}} | |||
| ⚫ | | |
||
| | PPhrases = {{P-phrases|261|264|270|271|273|280|301+312|302+352|304+340|305+351+338|312|330|332+313|337+313|362|403+233|405|501}} | |||
| ⚫ | | |
||
| | |
| NFPA-H = 2 | ||
| ⚫ | | NFPA-F = 1 | ||
| ⚫ | | NFPA-R = 0 | ||
| ⚫ | | NFPA-S = | ||
| }} | }} | ||
| }} | }} | ||
| '''2-Nitrobenzaldehyde''' is an ] with the formula {{chem2|O2NC6H4CHO}}. It is one of three isomers of ]. It contains a ] ] adjacent to the ].<ref>{{cite book |doi=10.1002/14356007.a03_463.pub2 |chapter=Benzaldehyde |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2011 |last1=Brühne |first1=Friedrich |last2=Wright |first2=Elaine |isbn=978-3-527-30385-4 }}</ref> | |||
| '''2-Nitrobenzaldehyde''' is an organic ] compound containing a ] ] ortho to ]. 2-Nitrobenzaldehyde once was produced as an intermediate in the synthesis of the popular dye ]. | |||
| ==Synthesis== | ==Synthesis== | ||
| The main routes to nitrobenzaldehyde begin with the nitration of ] |
The ] of ] produces mostly ]. Partly for this reason, 2-nitrobenzaldehyde is prepared by indirect routes.<ref>{{cite book |doi=10.1002/14356007.a03_463.pub2 |chapter=Benzaldehyde |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2011 |last1=Brühne |first1=Friedrich |last2=Wright |first2=Elaine |isbn=978-3-527-30385-4 }}</ref> The main routes to nitrobenzaldehyde begin with the nitration of ] or ] followed by the conversions of the resulting 2-nitrostyrene and 2-nitrocinnamic acids, respectively. ] can also be ] in high-yield to ].<ref>{{cite journal|journal=Org. Synth.|year=1953|volume=33|page=60|doi=10.15227/orgsyn.033.0060|title=o-Nitrocinnamaldehyde|author=Robert E. Buckles, M. Peter Bellis}}</ref> This compound is then oxidized to 2-nitrocinnamic acid, which is ] to the 2-nitrostyrene. The ] group can be oxidized in a number of different ways to yield 2-nitrobenzaldehyde.<ref>{{Cite journal |last1=Feng |first1=Bo |last2=Hou |first2=Zhenshan |last3=Wang |first3=Xiangrui |last4=Hu |first4=Yu |last5=Li |first5=Huan |last6=Qiao |first6=Yunxiang |date=2009-09-01 |title=Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water |url=https://pubs.rsc.org/en/content/articlelanding/2009/gc/b900807a |journal=Green Chemistry |language=en |volume=11 |issue=9 |pages=1446–1452 |doi=10.1039/B900807A |issn=1463-9270}}</ref> | ||
| 2-Nitrotoluene can be ] to yield 2-nitrobenzaldehyde.<ref>{{cite journal|journal=Acta Chim. Slov.|year=2005|volume=52|pages=460–462|title=Synthesis of 2-Nitrobenzaldehyde from 2-Nitrotoluene|author= | |||
| In one synthetic process, ] is mono-nitrated at cold temperatures to ], with about 58% being converted to the ortho- isomer, the remaining forming meta- and para- isomers.<ref>http://www.thecatalyst.org/experiments/AndersonS/AndersonS.html Product Distribution in the Nitration of Toluene, Steven W. Anderson, January 7, 1999</ref> The 2-nitrotoluene can then be ] to yield 2-nitrobenzaldehyde.<ref>, Alexander Popkov</ref><ref>{{cite web|title= o-Nitrobenzaldehyde|url=http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0641|work=|archiveurl=http://www.webcitation.org/5iUG3LUdZ|archivedate=2009-07-23|deadurl=no|accessdate=2009-07-21}}</ref> | |||
| Alexander Popkov}} {{Webarchive|url=https://web.archive.org/web/20110605084809/http://acta.chem-soc.si/52/52-4-460.pdf |date=2011-06-05 }}, Alexander Popkov</ref><ref>{{cite journal|journal=Org. Synth.|year=1944|volume=24|page=75|doi= 10.15227/orgsyn.024.0075|title= | |||
| o-Nitrobenzaldehyde|author1=S. M. Tsang |author2=Ernest H. Wood |author3=John R. Johnson}}</ref> | |||
| Alternatively, 2-nitrotoluene as formed above can be ] to a 2-nitrobenzyl halide followed by ] with ] and ] to yield 2-nitrobenzaldehyde, which is subsequently purified with the creation of a ] adduct.<ref>{{cite web|title=Process for the Preparation of 2-Nitrobenzaldehyde|url= |
Alternatively, 2-nitrotoluene as formed above can be ] to a 2-nitrobenzyl halide followed by ] with ] and ] to yield 2-nitrobenzaldehyde, which is subsequently purified with the creation of a ] adduct.<ref>{{cite web|title=Process for the Preparation of 2-Nitrobenzaldehyde|url=https://patents.google.com/patent/US4297519|access-date=2010-10-18}}</ref> | ||
| The nitration of ] produces mostly ], with yields being about 19% for the ortho-, 72% for the meta- and 9% for the para isomer.<ref></ref> For this reason, the nitration of benzaldehyde to yield 2-nitrobenzaldehyde is not cost-effective. | |||
| ==Uses== | ==Uses== | ||
| 2-Nitrobenzaldehyde is an intermediate in an early route to ], a water-insoluble dye commonly used to dye jeans and other fabrics. In the ], 2-nitrobenzaldehyde condenses with ] in basic aqueous solution to yield indigo in a one-pot synthesis |
2-Nitrobenzaldehyde is an intermediate in an early route to ], a water-insoluble dye commonly used to dye jeans and other fabrics. In the ], 2-nitrobenzaldehyde condenses with ] in basic aqueous solution to yield indigo in a one-pot synthesis<ref>{{Cite web |url=http://www.chem.missouri.edu/chem1100/Indigo%20synthesis.doc |title=Indigo Synthesis |access-date=2009-07-18 |archive-url=https://web.archive.org/web/20110720000154/http://www.chem.missouri.edu/chem1100/Indigo%20synthesis.doc |archive-date=2011-07-20 |url-status=dead }}</ref><ref>{{Cite web |url=http://courses.chem.psu.edu/chem36/Chem36H/IndivExpt1/863%20Indigo.pdf |title=Synthesis of Indigo and Vat Dyeing |access-date=2009-07-18 |archive-url=https://web.archive.org/web/20110720032713/http://courses.chem.psu.edu/chem36/Chem36H/IndivExpt1/863%20Indigo.pdf |archive-date=2011-07-20 |url-status=dead }}</ref> The method was abandoned in the early part of the 20th century, being replaced by routes from ].<ref>{{Cite book |url=https://onlinelibrary.wiley.com/doi/book/10.1002/14356007 |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2003-03-11 |publisher=Wiley |isbn=978-3-527-30385-4 |editor-last=Wiley-VCH |edition=1 |language=en |doi=10.1002/14356007.a14_149.pub2|chapter=Indigo and Indigo Colorants}}</ref> | ||
| :]{{clear-left}} | :]{{clear-left}} | ||
| Given its two relatively reactive groups, 2-nitrobenzaldehyde is a potential starting material for other compounds. Substituted 2-nitrobenzaldehydes can also be used to yield other important compounds based on indigo, such as ]. | Given its two relatively reactive groups, 2-nitrobenzaldehyde is a potential starting material for other compounds. Substituted 2-nitrobenzaldehydes can also be used to yield other important compounds based on indigo, such as ]. | ||
| 2-Nitrobenzaldehyde has been shown to be a useful ] for various |
2-Nitrobenzaldehyde has been shown to be a useful ] for various functions.<ref>{{cite journal|title=Photochemistry of 2-Nitrobenzylidene Acetals|first1=Peter|last1=Šebej|first2=Tomáš|last2=Šolomek|first3=Ľubica|last3=Hroudná|first4=Pavla|last4=Brancová|first5=Petr|last5=Klán|journal=J. Org. Chem.|year=2009|volume=74|issue=22|pages=8647–8658|doi=10.1021/jo901756r|pmid=19824651}}</ref><ref>{{cite journal|title=Chemical Actinometry: Using o-Nitrobenzaldehyde to Measure Lamp Intensity in Photochemical Experiments|author1=Kristine L. Willett |author2=Ronald A. Hites|journal=J. Chem. Educ.|year=2000|volume=77|issue=7 |pages=900|doi=10.1021/ed077p900|bibcode=2000JChEd..77..900W }}</ref> | ||
| ==References== | ==References== | ||
| Line 66: | Line 76: | ||
| {{DEFAULTSORT:Nitrobenzaldehyde, 2-}} | {{DEFAULTSORT:Nitrobenzaldehyde, 2-}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 17:32, 17 November 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Nitrobenzaldehyde | |||
| Other names
Nitrobenzaldehyde ortho-Nitrobenzaldehyde o-Nitrobenzaldehyde | |||
| Identifiers | |||
| CAS Number | |||
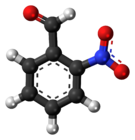
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.206 | ||
| EC Number |
| ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H5NO3 | ||
| Molar mass | 151.12 g/mol | ||
| Appearance | Pale yellow crystalline powder | ||
| Melting point | 43 °C (109 °F; 316 K) | ||
| Boiling point | 152 °C (306 °F; 425 K) | ||
| Solubility in water | Insoluble | ||
| Magnetic susceptibility (χ) | -68.23·10 cm/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Harmful, Potentially mutagenic | ||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H315, H319, H335, H412 | ||
| Precautionary statements | P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
2-Nitrobenzaldehyde is an organic compound with the formula O2NC6H4CHO. It is one of three isomers of nitrobenzaldehyde. It contains a nitro group adjacent to the formyl group.
Synthesis
The nitration of benzaldehyde produces mostly 3-nitrobenzaldehyde. Partly for this reason, 2-nitrobenzaldehyde is prepared by indirect routes. The main routes to nitrobenzaldehyde begin with the nitration of styrene or cinnamic acid followed by the conversions of the resulting 2-nitrostyrene and 2-nitrocinnamic acids, respectively. Cinnamaldehyde can also be nitrated in high-yield to 2-nitrocinnamaldehyde. This compound is then oxidized to 2-nitrocinnamic acid, which is decarboxylated to the 2-nitrostyrene. The vinyl group can be oxidized in a number of different ways to yield 2-nitrobenzaldehyde.
2-Nitrotoluene can be oxidized to yield 2-nitrobenzaldehyde.
Alternatively, 2-nitrotoluene as formed above can be halogenated to a 2-nitrobenzyl halide followed by oxidation with DMSO and sodium bicarbonate to yield 2-nitrobenzaldehyde, which is subsequently purified with the creation of a bisulfite adduct.
Uses
2-Nitrobenzaldehyde is an intermediate in an early route to indigo, a water-insoluble dye commonly used to dye jeans and other fabrics. In the Baeyer-Drewson indigo synthesis, 2-nitrobenzaldehyde condenses with acetone in basic aqueous solution to yield indigo in a one-pot synthesis The method was abandoned in the early part of the 20th century, being replaced by routes from aniline.
Given its two relatively reactive groups, 2-nitrobenzaldehyde is a potential starting material for other compounds. Substituted 2-nitrobenzaldehydes can also be used to yield other important compounds based on indigo, such as indigo carmine.
2-Nitrobenzaldehyde has been shown to be a useful photoremovable protecting group for various functions.
References
- "2-Nitrobenzaldehyde". pubchem.ncbi.nlm.nih.gov.
- Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3-527-30385-4.
- Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3-527-30385-4.
- Robert E. Buckles, M. Peter Bellis (1953). "o-Nitrocinnamaldehyde". Org. Synth. 33: 60. doi:10.15227/orgsyn.033.0060.
- Feng, Bo; Hou, Zhenshan; Wang, Xiangrui; Hu, Yu; Li, Huan; Qiao, Yunxiang (2009-09-01). "Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water". Green Chemistry. 11 (9): 1446–1452. doi:10.1039/B900807A. ISSN 1463-9270.
- Alexander Popkov (2005). "Synthesis of 2-Nitrobenzaldehyde from 2-Nitrotoluene". Acta Chim. Slov. 52: 460–462. Archived 2011-06-05 at the Wayback Machine, Alexander Popkov
- S. M. Tsang; Ernest H. Wood; John R. Johnson (1944). "o-Nitrobenzaldehyde". Org. Synth. 24: 75. doi:10.15227/orgsyn.024.0075.
- "Process for the Preparation of 2-Nitrobenzaldehyde". Retrieved 2010-10-18.
- "Indigo Synthesis". Archived from the original on 2011-07-20. Retrieved 2009-07-18.
- "Synthesis of Indigo and Vat Dyeing" (PDF). Archived from the original (PDF) on 2011-07-20. Retrieved 2009-07-18.
- Wiley-VCH, ed. (2003-03-11). "Indigo and Indigo Colorants". Ullmann's Encyclopedia of Industrial Chemistry (1 ed.). Wiley. doi:10.1002/14356007.a14_149.pub2. ISBN 978-3-527-30385-4.
- Šebej, Peter; Šolomek, Tomáš; Hroudná, Ľubica; Brancová, Pavla; Klán, Petr (2009). "Photochemistry of 2-Nitrobenzylidene Acetals". J. Org. Chem. 74 (22): 8647–8658. doi:10.1021/jo901756r. PMID 19824651.
- Kristine L. Willett; Ronald A. Hites (2000). "Chemical Actinometry: Using o-Nitrobenzaldehyde to Measure Lamp Intensity in Photochemical Experiments". J. Chem. Educ. 77 (7): 900. Bibcode:2000JChEd..77..900W. doi:10.1021/ed077p900.