| Revision as of 15:01, 13 February 2012 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'CASNo_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|er...← Previous edit | Latest revision as of 09:17, 27 September 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,127 edits removed Category:Nitrobenzene derivatives; added Category:4-Nitrophenyl compounds using HotCat | ||
| (35 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | | verifiedrevid = 477314571 | ||
| ⚫ | | |
||
| ⚫ | | Name = Alizarine Yellow R | ||
| ⚫ | | |
||
| | |
| ImageFile1 = Alizarin-yellow-R-1718-34-9-sodium-salt-2D-skeletal.png | ||
| ⚫ | | ImageSize1 = 220px | ||
| | ImageFile2 = Alizarin-yellow-R-2243-76-7-acid-2D-skeletal.png | |||
| | ImageName1 = Alizarin Yellow R (sodium salt) | |||
| ⚫ | | |
||
| | |
| ImageFile2 = Alizarine-Yellow-R-sodium-3D-spacefill.png | ||
| | ImageAlt2 = Space-filling model of Alizarine Yellow R as a sodium salt | |||
| | IUPACName = | |||
| ⚫ | | ImageFile3 = Alizarin-yellow-R-2243-76-7-acid-2D-skeletal.png | ||
| ⚫ | | OtherNames = 5-salicylic acid sodium salt |
||
| ⚫ | | ImageSize3 = 200px | ||
| | ImageName3 = Alizarin Yellow R (acid) | |||
| | IUPACName = Sodium 2-hydroxy-5-benzoate | |||
| ⚫ | | OtherNames = 5-salicylic acid sodium salt<br />Chrome orange<br />Mordant orange 1<br />C.I. 14030 | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 17215762 | | ChemSpiderID = 17215762 | ||
| | InChI = 1/C13H9N3O5.Na/c17-12-6-3-9(7-11(12)13(18)19)15-14-8-1-4-10(5-2-8)16(20)21;/h1-7,17H,(H,18,19);/q;+1/p-1/b15-14+; | | InChI = 1/C13H9N3O5.Na/c17-12-6-3-9(7-11(12)13(18)19)15-14-8-1-4-10(5-2-8)16(20)21;/h1-7,17H,(H,18,19);/q;+1/p-1/b15-14+; | ||
| Line 19: | Line 23: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = HXKKTXMJSVFQSL-WPDLWGESSA-M | | StdInChIKey = HXKKTXMJSVFQSL-WPDLWGESSA-M | ||
| | CASNo_Ref = {{cascite|correct| |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 1718-34-9 | | CASNo = 1718-34-9 | ||
| | CASNo_Comment =(Na salt) | |||
| | CASOther = (Na salt)<br />2243-76-7 (acid)<ref name="CRC">{{ cite book | title = CRC Handbook of Chemistry and Physics, 89th Edition | last = Lide | first = David R. | year = 2008 | publisher = ] | isbn = 978-0-8493-0488-0 | pages = 3–10}}</ref> | |||
| | CASNo2_Ref = {{cascite|correct|CAS}} | |||
| ⚫ | | |
||
| | CASNo2 = 2243-76-7 | |||
| | CASNo2_Comment = (acid)<ref name="CRC">{{cite book|title=CRC Handbook of Chemistry and Physics, 88th Edition|url=https://books.google.com/books?id=SdW_QgAACAAJ|url-access =limited|last=Lide|first=David R.|date=25 June 2007|publisher=]|oclc=1024315229|isbn=9780849304880|pages=–10}}</ref> | |||
| | PubChem = 6504724 | |||
| | PubChem_Comment =(Na salt) | |||
| | EC_number = 209-536-1 | |||
| | UNII1_Ref = {{fdacite|correct|FDA}} | |||
| | UNII1 = R1T3O0G585 | |||
| | UNII1_Comment = (Na salt) | |||
| | UNII2_Ref = {{fdacite|correct|FDA}} | |||
| | UNII2 = OBF2VZO457 | |||
| | UNII2_Comment = (acid) | |||
| ⚫ | | SMILES = .O=C()c1cc(ccc1O)/N=N/c2ccc(cc2)()=O | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = C<sub>13</sub>H<sub>8</sub>N<sub>3</sub>NaO<sub>5</sub> (Na salt)<br />C<sub>13</sub>H<sub>9</sub>N<sub>3</sub>O<sub>5</sub> (acid) | ||
| | |
| MolarMass = 309.21 g mol<sup>−1</sup> (Na salt) <br /> 287.23 g mol<sup>−1</sup> (acid) | ||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | FlashPt = | |||
| | MainHazards = Harmful | |||
| | |
| AutoignitionPt = | ||
| | GHSPictograms = {{GHS exclamation mark}} | |||
| | Autoignition = | |||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|H302|H319}} | |||
| | PPhrases = {{P-phrases|P264|P270|P280|P301+P312|P305+P351+P338|P330|P337+P313|P501}} | |||
| | Hazards_ref = {{Sigma-Aldrich|id=R320897|name=ALIZARINE YELLOW R|accessdate=09 April 2023}} | |||
| }} | }} | ||
| }} | }} | ||
| ⚫ | {{pH indicator template|indicator_name=Alizarine Yellow R|low_pH=10.1|high_pH=12.0|low_pH_color=yellow|high_pH_color=red|high_pH_text=white}} | ||
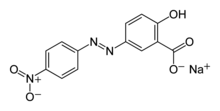
| '''Alizarine Yellow R''' is a ] colored ] made by the ] ]. It is usually commercially available as a sodium salt. In its pure form, it is a rust-colored solid.<ref>{{cite web|url=http://msds.chem.ox.ac.uk/AL/alizarin_yellow_R.html|url-status=dead|title=Safety Datasheet (MSDS) for alizarin yellow R|archive-url=https://web.archive.org/web/20110319233005/http://msds.chem.ox.ac.uk/AL/alizarin_yellow_R.html|date=2005|access-date=11 October 2008|archive-date=19 March 2011|publisher=Department of Chemistry, University of Oxford}}</ref> It is mainly used as a ]. | |||
| ==Preparation== | |||
| '''Alizarine Yellow R''' is a ] colored ] made by the ] ]. It usually comes as a sodium salt. In its pure form it is a rust-colored solid.<ref>http://msds.chem.ox.ac.uk/AL/alizarin_yellow_R.html</ref> | |||
| Alizarine Yellow R is produced by ] of salicylic ] and diazonium derivative of ] | |||
| ] | |||
| ==Special properties== | |||
| Alizarine Yellow R is a ]. | |||
| ⚫ | {{pH indicator template|indicator_name=Alizarine Yellow R|low_pH=10.1|high_pH=12.0|low_pH_color=yellow|high_pH_color=red}} | ||
| {{clear}} | |||
| ==References== | ==References== | ||
| {{Reflist}} | |||
| <references/> | |||
| ==External links== | |||
| * | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| {{organic-compound-stub}} | {{organic-compound-stub}} | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 09:17, 27 September 2024
| |

| |

| |
| Names | |
|---|---|
| IUPAC name Sodium 2-hydroxy-5-benzoate | |
| Other names
5-salicylic acid sodium salt Chrome orange Mordant orange 1 C.I. 14030 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.017.109 |
| EC Number |
|
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C13H8N3NaO5 (Na salt) C13H9N3O5 (acid) |
| Molar mass | 309.21 g mol (Na salt) 287.23 g mol (acid) |
| HazardsSigma-Aldrich Co., ALIZARINE YELLOW R. Retrieved on 09 April 2023. | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
| Alizarine Yellow R (pH indicator) | ||
| below pH 10.1 | above pH 12.0 | |
| 10.1 | ⇌ | 12.0 |
Alizarine Yellow R is a yellow colored azo dye made by the diazo coupling reaction. It is usually commercially available as a sodium salt. In its pure form, it is a rust-colored solid. It is mainly used as a pH indicator.
Preparation
Alizarine Yellow R is produced by azo coupling of salicylic acid and diazonium derivative of 4-Nitroaniline
References
- Lide, David R. (25 June 2007). CRC Handbook of Chemistry and Physics, 88th Edition. CRC Press. pp. 3–10. ISBN 9780849304880. OCLC 1024315229.
- "Safety Datasheet (MSDS) for alizarin yellow R". Department of Chemistry, University of Oxford. 2005. Archived from the original on 19 March 2011. Retrieved 11 October 2008.
External links
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |
