| Revision as of 12:34, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473657671 of page Gallium_nitride for the Chem/Drugbox validation project (updated: '').← Previous edit |
Latest revision as of 14:25, 29 January 2022 edit undo119.246.116.98 (talk)No edit summary |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|drugbox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{chembox |
|
{{drugbox |
|
|
| verifiedrevid = 477001424 |
|
| Watchedfields = changed |
|
|
|
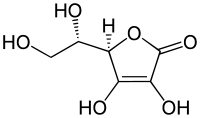
| IUPAC_name = 2-Oxo-<small>L</small>-threo-hexono-1,4-lactone-2,3-enediol<br />''or''<br />(''R'')-3,4-dihydroxy-5-((''S'')- 1,2-dihydroxyethyl)furan-2(5''H'')-one |
|
| verifiedrevid = 450764160 |
|
|
|
| image = L-Ascorbic_acid.svg |
|
| Name = Gallium nitride |
|
|
|
| width = 200px |
|
| ImageFile = GaNcrystal.jpg |
|
|
|
| image2 = Ascorbic-acid-from-xtal-1997-3D-balls.png |
|
| ImageFile2 = Wurtzite polyhedra.png |
|
|
|
| width2 = 200px |
|
| IUPACName = Gallium nitride |
|
|
|
|
|
| OtherNames = |
|
|
|
<!--Clinical data--> |
|
| Name = |
|
|
|
| Drugs.com = {{drugs.com|MTM|vitamin_c}} |
|
| Section1 = {{Chembox Identifiers |
|
|
|
| licence_EU = <!-- EMEA requires brand name --> |
|
|
| licence_US = <!-- FDA may use generic name --> |
|
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
|
| pregnancy_category = A |
|
|
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
|
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
|
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
|
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
|
| legal_status = general public availability |
|
|
| routes_of_administration = oral |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
|
| bioavailability = rapid & complete |
|
|
| protein_bound = negligible |
|
|
| elimination_half-life = varies according to plasma concentration <!-- can be 30 min to weeks, depending on body stores --> |
|
|
| excretion = renal |
|
|
|
|
|
<!--Identifiers--> |
|
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
|
| CAS_number = 50-81-7 |
|
|
| ATC_prefix = A |
|
|
| ATC_suffix = 11G |
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEBI = 29073 |
|
|
| PubChem = 5785 |
|
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
|
| DrugBank = DB00126 |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 105057 |
|
| ChemSpiderID = 10189562 |
|
|
| NIAID_ChemDB = 002072 |
|
| InChI = 1/Ga.N/rGaN/c1-2 |
|
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| SMILES = #N |
|
|
|
| UNII = PQ6CK8PD0R |
|
| InChIKey = JMASRVWKEDWRBT-MDMVGGKAAI |
|
|
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
|
| KEGG = D00018 |
|
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEMBL = 196 |
|
|
|
|
|
<!--Chemical data--> |
|
|
| chemical_formula = |
|
|
| C=6 | H=7 | O=6 |
|
|
| molecular_weight = 176.12 g/] |
|
|
| smiles = C((1C(=C(C(=O)O1)O)O)O)O |
|
|
| InChI = 1/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1 |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
|
| StdInChI = 1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1 |
|
| StdInChI = 1S/Ga.N |
|
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = JMASRVWKEDWRBT-UHFFFAOYSA-N |
|
| StdInChIKey = CIWBSHSKHKDKBQ-JLAZNSOCSA-N |
|
|
| synonyms = <small>L</small>-ascorbic acid |
|
| CASNo = 25617-97-4 |
|
|
|
| density = 1.694 |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| PubChem = 117559 |
|
| melting_point = 190 |
|
|
| boiling_point = 553 |
|
| RTECS = LW9640000 |
|
|
}} |
|
|
| Section2 = {{Chembox Properties |
|
|
| Formula = GaN |
|
|
| MolarMass = 83.73 g/mol |
|
|
| Appearance = yellow powder |
|
|
| Density = 6.15 g/cm<sup>3</sup> |
|
|
| Solubility = Reacts. |
|
|
| MeltingPt = >2500°C<ref name=melting>{{cite journal| author = T. Harafuji and J. Kawamura|title = Molecular dynamics simulation for evaluating melting point of wurtzite-type GaN crystal| doi = 10.1063/1.1772878|journal = Appl. Phys.|volume = 96| page = 2501|year = 2004| issue = 5}}</ref> |
|
|
| BoilingPt = |
|
|
| pKb = |
|
|
| BandGap = 3.4 eV (300 K, direct) |
|
|
| ElectronMobility = 440 cm<sup>2</sup>/(V·s) (300 K) |
|
|
| ThermalConductivity = 2.3 W/(cm·K) (300 K) <ref>Mion, Christian. "Investigation of the Thermal Properties of Gallium |
|
|
Nitride Using the Three Omega Technique." Diss. North Carolina State University. Raleigh, 2005. Web, Aug 12, 2011. http://repository.lib.ncsu.edu/ir/bitstream/1840.16/5418/1/etd.pdf.</ref> |
|
|
| RefractIndex = 2.429 |
|
|
}} |
|
|
| Section3 = {{Chembox Structure |
|
|
| CrystalStruct = ] |
|
|
| SpaceGroup = ''C''<sub>6v</sub><sup>4</sup>-''P''6<sub>3</sub>''mc'' |
|
|
| Coordination = Tetrahedral |
|
|
| LattConst_a = 3.186 Å |
|
|
| LattConst_c = 5.186 Å <ref>Bougrov V., Levinshtein M.E., Rumyantsev S.L., Zubrilov A., in ''Properties of Advanced Semiconductor Materials GaN, AlN, InN, BN, SiC, SiGe''. Eds. Levinshtein M.E., Rumyantsev S.L., Shur M.S., John Wiley & Sons, Inc., New York, 2001, 1–30</ref> |
|
|
}} |
|
|
| Section7 = {{Chembox Hazards |
|
|
| EUIndex = Not listed |
|
|
| RPhrases = |
|
|
| SPhrases = |
|
|
| FlashPt = Non-flammable. |
|
|
}} |
|
|
| Section8 = {{Chembox Related |
|
|
| OtherAnions = ]<br/>]<br/>] |
|
|
| OtherCations = ]<br/>]<br/>] |
|
|
| Function = |
|
|
| OtherFunctn = |
|
|
| OtherCpds = ]<br/>]<br/>]<br/>]<br/>] |
|
|
}} |
|
|
}} |
|
}} |