| Revision as of 19:50, 16 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Saving copy of the {{chembox}} taken from revid 477101262 of page Acetic_acid for the Chem/Drugbox validation project (updated: 'KEGG').← Previous edit |
Latest revision as of 14:25, 29 January 2022 edit undo119.246.116.98 (talk)No edit summary |
| (757 intermediate revisions by 8 users not shown) |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|drugbox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{Chembox |
|
{{drugbox |
|
|
| verifiedrevid = 477001424 |
|
| Verifiedfields = changed |
|
|
|
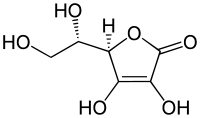
| IUPAC_name = 2-Oxo-<small>L</small>-threo-hexono-1,4-lactone-2,3-enediol<br />''or''<br />(''R'')-3,4-dihydroxy-5-((''S'')- 1,2-dihydroxyethyl)furan-2(5''H'')-one |
|
| verifiedrevid = 476998154 |
|
|
|
| image = L-Ascorbic_acid.svg |
|
| ImageFile3 = Acetic acid.jpg |
|
|
|
| width = 200px |
|
| ImageFile3_Ref = {{chemboximage|correct|??}} |
|
|
|
| image2 = Ascorbic-acid-from-xtal-1997-3D-balls.png |
|
| ImageSize3 = 244 |
|
|
|
| width2 = 200px |
|
| ImageName3 = Sample of acetic acid in a reagent bottle |
|
|
|
|
|
| ImageFileL1 = Acetic-acid-2D-skeletal.svg |
|
|
|
<!--Clinical data--> |
|
| ImageFileL1_Ref = {{chemboximage|correct|??}} |
|
|
|
| Drugs.com = {{drugs.com|MTM|vitamin_c}} |
|
| ImageSizeL1 = 121 |
|
|
|
| licence_EU = <!-- EMEA requires brand name --> |
|
| ImageNameL1 = Skeletal formula of acetic acid |
|
|
|
| licence_US = <!-- FDA may use generic name --> |
|
| ImageFileR1 = Acetic-acid-CRC-GED-3D-vdW-B.png |
|
|
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| ImageFileR1_Ref = {{chemboximage|correct|??}} |
|
|
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| ImageSizeR1 = 121 |
|
|
|
| pregnancy_category = A |
|
| ImageNameR1 = Spacefill model of acetic acid |
|
|
|
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
| ImageFileL2 =Acetic-acid-2D-flat.png |
|
|
|
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
| ImageFileL2_Ref = {{chemboximage|correct|??}} |
|
|
|
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
| ImageSizeL2 = 121 |
|
|
|
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| ImageNameL2 = Skeletal formula of acetic acid with all explicit hydogens added |
|
|
|
| legal_status = general public availability |
|
| ImageFileR2 = Acetic-acid-CRC-GED-3D-balls-B.png |
|
|
|
| routes_of_administration = oral |
|
| ImageFileR2_Ref = {{chemboximage|correct|??}} |
|
|
|
|
|
| ImageSizeR2 = 121 |
|
|
|
<!--Pharmacokinetic data--> |
|
| ImageNameR2 = Ball and stick model of acetic acid |
|
|
|
| bioavailability = rapid & complete |
|
| IUPACName = Acetic acid<ref>{{cite book | title=A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993) | publisher=Blackwell Scientific publications | author=IUPAC, Commission on Nomenclature of Organic Chemistry | year=1993 | chapter = Table 28(a) Carboxylic acids and related groups.Unsubstituted parent structures | url = http://www.acdlabs.eu/iupac/nomenclature/93/r93_705.htm }}</ref><ref>{{Cite web|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=176|title = Acetic Acid - PubChem Public Chemical Database|work = The PubChem Project|location = USA|publisher = National Center for Biotechnology Information}}</ref> |
|
|
|
| protein_bound = negligible |
|
| SystematicName = Ethanoic acid<ref name="BB-prs310305"/> |
|
|
|
| elimination_half-life = varies according to plasma concentration <!-- can be 30 min to weeks, depending on body stores --> |
|
| OtherNames = Methanecarboxylic acid<ref>{{cite book|title=Scientific literature reviews on generally recognized as safe (GRAS) food ingredients|publisher=National Technical Information Service|year=1974|page=1}}</ref><ref>"Chemistry", volume 5, Encyclopedia Britannica, 1961, page 374</ref> |
|
|
|
| excretion = renal |
|
| Section1 = {{Chembox Identifiers |
|
|
|
|
|
| Abbreviations = AcOH |
|
|
|
<!--Identifiers--> |
|
| CASNo = 64-19-7 |
|
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| PubChem = 176 |
|
|
|
| CAS_number = 50-81-7 |
|
| PubChem_Ref = {{Pubchemcite|correct|pubchem}} |
|
|
| ChemSpiderID = 171 |
|
| ATC_prefix = A |
|
|
| ATC_suffix = 11G |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
| UNII = Q40Q9N063P |
|
|
|
| ChEBI = 29073 |
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| EINECS = 200-580-7 |
|
| PubChem = 5785 |
|
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| UNNumber = 2789 |
|
|
|
| DrugBank = DB00126 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
|
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| DrugBank = DB03166 |
|
|
|
| ChemSpiderID = 10189562 |
|
| KEGG = <!-- blanked - oldvalue: D00010 --> |
|
|
|
| NIAID_ChemDB = 002072 |
|
| KEGG_Ref = {{keggcite|changed|kegg}} |
|
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| MeSHName = Acetic+acid |
|
|
|
| UNII = PQ6CK8PD0R |
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
|
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| ChEBI = 15366 |
|
|
| ChEMBL = 539 |
|
| KEGG = D00018 |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| IUPHAR_ligand = 1058 |
|
| ChEMBL = 196 |
|
|
|
|
| RTECS = AF1225000 |
|
|
|
<!--Chemical data--> |
|
| ATCCode_prefix = G01 |
|
|
|
| chemical_formula = |
|
| ATCCode_suffix = AD02 |
|
|
|
| C=6 | H=7 | O=6 |
|
| ATC_Supplemental = {{ATC|S02|AA10}} |
|
|
|
| molecular_weight = 176.12 g/] |
|
| Beilstein = 506007 |
|
|
|
| smiles = C((1C(=C(C(=O)O1)O)O)O)O |
|
| Gmelin = 1380 |
|
|
|
| InChI = 1/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1 |
|
| 3DMet = B00009 |
|
|
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| SMILES = CC(O)=O |
|
|
| StdInChI = 1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4) |
|
| StdInChI = 1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1 |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = QTBSBXVTEAMEQO-UHFFFAOYSA-N |
|
| StdInChIKey = CIWBSHSKHKDKBQ-JLAZNSOCSA-N |
|
|
| synonyms = <small>L</small>-ascorbic acid |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
|
|
| density = 1.694 |
|
}} |
|
|
|
| melting_point = 190 |
|
| Section2 = {{Chembox Properties |
|
|
| C = 2 |
|
| boiling_point = 553 |
|
| H = 4 |
|
|
| O = 2 |
|
|
| ExactMass = 60.021129372 g mol<sup>-1</sup> |
|
|
| Appearance = Colourless liquid |
|
|
| Density = 1.049 g cm<sup>-3</sup> |
|
|
| Solubility = Miscible |
|
|
| MeltingPtKL = 289 |
|
|
| MeltingPtKH = 290 |
|
|
| BoilingPtKL = 391 |
|
|
| BoilingPtKH = 392 |
|
|
| LogP = -0.322 |
|
|
| pKa = 4.792 |
|
|
| pKb = 9.198 |
|
|
| Viscosity = 1.22 mPa s |
|
|
| Dipole = 1.74 D |
|
|
}} |
|
|
| Section4 = {{Chembox Thermochemistry |
|
|
| DeltaHf = -483.88--483.16 kJ mol<sup>-1</sup> |
|
|
| DeltaHc = -875.50--874.82 kJ mol<sup>-1</sup> |
|
|
| Entropy = 158.0 J K<sup>-1</sup> mol<sup>-1</sup> |
|
|
| HeatCapacity = 123.1 J K<sup>-1</sup> mol<sup>-1</sup> |
|
|
}} |
|
|
| Section5 = {{Chembox Hazards |
|
|
| GHSPictograms = {{GHS02}} {{GHS05}} |
|
|
| GHSSignalWord = Danger |
|
|
| HPhrases = {{H-phrases|226|314}} |
|
|
| PPhrases = {{P-phrases|280|305+351+338|310}} |
|
|
| EUIndex = 607-002-00-6 |
|
|
| EUClass = {{Hazchem C}} |
|
|
| RPhrases = {{R10}}, {{R35}} |
|
|
| SPhrases = {{S1/2}}, {{S23}}, {{S26}}, {{S45}} |
|
|
| NFPA-F = 2 |
|
|
| NFPA-H = 3 |
|
|
| NFPA-R = 1 |
|
|
| FlashPt = 40 °C |
|
|
| Autoignition = 400 °C |
|
|
| LD50 = 3.31 g kg<sup>-1</sup>, oral (rat) |
|
|
}} |
|
|
| Section6 = {{Chembox Related |
|
|
| Function = ]s |
|
|
| OtherFunctn = ]<br />] |
|
|
| OtherCpds = ]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
]<br /> |
|
|
] |
|
|
}} |
|
|
}} |
|
}} |