| Revision as of 06:52, 5 March 2012 editPlasmic Physics (talk | contribs)Extended confirmed users, Rollbackers19,174 editsNo edit summary← Previous edit | Latest revision as of 13:57, 9 April 2024 edit undoAumur (talk | contribs)2 editsm neopentane (2,2-dimethylpropane)Tag: Visual edit | ||
| (77 intermediate revisions by 53 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| | verifiedrevid = 464398176 | | verifiedrevid = 464398176 | ||
| | ImageFile = |
| ImageFile = Isopentane-2D-skeletal.svg | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | | ImageFile_Ref = {{chemboximage|correct|??}} | ||
| | ImageSize = 100 | | ImageSize = 100 | ||
| | ImageName = |
| ImageName = Skeletal formula of isopentane | ||
| | ImageFile1 = Isopentane |
| ImageFile1 = Isopentane.PNG | ||
| | ImageFile1_Ref = {{chemboximage|correct|??}} | | ImageFile1_Ref = {{chemboximage|correct|??}} | ||
| | ImageSize1 = |
| ImageSize1 = 160 | ||
| | ImageName1 = Skeletal formula of isopentane with all implicit carbons shown, and all explicit hydrogens added | |||
| | ImageName1 = Ball and stick model of isopentane | |||
| | ImageFile2 = Isopentane-3D-balls.png | |||
| | IUPACName = 2-Methylbutane<ref>{{Cite web|title=isopentane - Compound Summary|url=http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6556&loc=ec_rcs#x291|work=PubChem Compound|publisher=National Center for Biotechnology Information|accessdate=5 March 2012|location=USA|date=16 September 2004|at=Identification and Related Records}}</ref> | |||
| | ImageFile2_Ref = {{chemboximage|correct|??}} | |||
| | OtherNames = Methylbutane{{Citation needed|date=March 2012}} | |||
| | ImageSize2 = 100 | |||
| | Section1 = {{Chembox Identifiers | |||
| | ImageName2 = Ball and stick model of isopentane | |||
| | CASNo = 78-78-4 | |||
| | PIN = 2-Methylbutane<ref name="IUPAC2013_652">{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = ] | date = 2014 | location = Cambridge | page = 652 | doi = 10.1039/9781849733069-FP001 | isbn = 978-0-85404-182-4 | quote = The names ‘isobutane’, ‘isopentane’ and ‘neopentane’ are no longer recommended.}}</ref> | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | OtherNames = Isopentane | |||
| | PubChem = 6556 | |||
| |Section1={{Chembox Identifiers | |||
| | PubChem_Ref = {{Pubchemcite|correct|Pubchem}} | |||
| | CASNo = 78-78-4 | |||
| | ChemSpiderID = 6308 | |||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| PubChem = 6556 | ||
| | ChemSpiderID = 6308 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | EINECS = 201-142-8 | |||
| | |
| UNII = ZH67814I0O | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | MeSHName = isopentane | |||
| | |
| EINECS = 201-142-8 | ||
| | UNNumber = 1265 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | |
| MeSHName = isopentane | ||
| | |
| ChEBI = 30362 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | Gmelin = 49318 | |||
| | |
| RTECS = EK4430000 | ||
| | Beilstein = 1730723 | |||
| | StdInChI = 1S/C5H12/c1-4-5(2)3/h5H,4H2,1-3H3 | |||
| | Gmelin = 49318 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | SMILES = CCC(C)C | |||
| | StdInChIKey = QWTDNUCVQCZILF-UHFFFAOYSA-N | |||
| | StdInChI = 1S/C5H12/c1-4-5(2)3/h5H,4H2,1-3H3 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = QWTDNUCVQCZILF-UHFFFAOYSA-N | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=5 | H=12 | ||
| | Appearance = Colorless liquid | |||
| | H = 12 | |||
| | Odor = Gasoline-like | |||
| | ExactMass = 72.093900384 g mol<sup>−1</sup> | |||
| | Density = 616 mg mL<sup>−1</sup><ref name="Wei">James Wei (1999), ''Molecular Symmetry, Rotational Entropy, and Elevated Melting Points''. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 {{doi|10.1021/ie990588m}}</ref> | |||
| | Appearance = Colorless, transparent liquid | |||
| | BoilingPtK = 300.9 to 301.3 | |||
| | Odor = Odorless | |||
| | MeltingPtK = 112 to 114 | |||
| | Density = 616 mg mL<sup>−1</sup><ref name="Wei"> James Wei (1999), ''Molecular Symmetry, Rotational Entropy, and Elevated Melting Points''. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 {{doi|10.1021/ie990588m}}</ref> | |||
| | VaporPressure = 76.992 kPa (at 20 °C) | |||
| | BoilingPtKL = 300.9 | |||
| | HenryConstant = 7.2 nmol Pa<sup>−1</sup> kg<sup>−1</sup> | |||
| | BoilingPtKH = 301.3 | |||
| | |
| LambdaMax = 192 nm | ||
| | |
| RefractIndex = 1.354 | ||
| | Viscosity = 0.214 cP (at 20 °C) | |||
| | Solubility = Miscible | |||
| | VaporPressure = 76.992 kPa (at 20 °C) | |||
| | HenryConstant = 7.2 nmol Pa<sup>−1</sup> kg<sup>−1</sup> | |||
| | LambdaMax = 192 nm | |||
| | RefractIndex = 10.354 | |||
| }} | }} | ||
| | |
|Section4={{Chembox Thermochemistry | ||
| | |
| DeltaHf = −179.1–−177.3 kJ mol<sup>−1</sup> | ||
| | |
| DeltaHc = ~ 3.3 MJ mol<sup>−1</sup>, 19,664 Btu/lb | ||
| | |
| Entropy = 260.41 J K<sup>−1</sup> mol<sup>−1</sup> | ||
| | |
| HeatCapacity = 164.85 J K<sup>−1</sup> mol<sup>−1</sup> | ||
| }} | }} | ||
| | |
|Section5={{Chembox Hazards | ||
| | |
| GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} {{GHS health hazard}} {{GHS environment}} | ||
| | |
| GHSSignalWord = '''DANGER''' | ||
| | |
| HPhrases = {{H-phrases|224|301|302|305|336|411}} | ||
| | |
| PPhrases = {{P-phrases|210|261|273|301+310|331}} | ||
| | |
| NFPA-H = 1 | ||
| | NFPA-F = 4 | |||
| | EUClass = {{Hazchem F+}} {{Hazchem Xn}} {{Hazchem N}} | |||
| | |
| NFPA-R = 0 | ||
| | |
| FlashPtC = −51 | ||
| | |
| AutoignitionPtC = 420 | ||
| | ExploLimits = 1.4–8.3% | |||
| | RPhrases = {{R12}}, {{R51/53}}, {{R65}}, {{R66}}, {{R67}} | |||
| | SPhrases = {{S2}}, {{S16}}, {{S29}}, {{S33}} | |||
| | FlashPt = −51 °C | |||
| | Autoignition = 420 °C | |||
| | ExploLimits = 1.4–8.3% | |||
| }} | }} | ||
| | |
|Section6={{Chembox Related | ||
| | |
| OtherFunction_label = alkanes | ||
| | |
| OtherFunction = {{Unbulleted list|]|]|]|]}} | ||
| | OtherCompounds = ] | |||
| }} | }} | ||
| }} | }} | ||
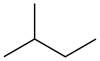
| '''Isopentane''', also called '''methylbutane''' or '''2-methylbutane''', is a branched-chain saturated ] (an ]) with five ] atoms, with formula {{chem|C|5|H|12}} or {{chem|CH(CH|3|)|2|(C|2|H|5|)}}. | |||
| '''Isopentane''', ]], also called '''methylbutane''' or '''2-methylbutane''', is a branched-chain ] with five ] atoms. Isopentane is an extremely ] and extremely ] liquid at room ] and ]. The ] is just a few degrees above room temperature and isopentane will readily boil and evaporate away on a warm day. Isopentane is commonly used in conjunction with ] to achieve a liquid bath temperature of -160 °C. It is 1% or less of natural gas.<ref>Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme “Natural Gas“ in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a17_073.pub2}}</ref> | |||
| Isopentane is a ] and ] liquid. It is one of three ] with the ] C<sub>5</sub>H<sub>12</sub>, the others being ] (''n''-pentane) and ] (2,2-dimethylpropane). | |||
| ==Nomenclature== | |||
| Isopentane is the name recommended by the ] (IUPAC).<ref>{{cite book | author=Panico, R.; & Powell, W. H. (Eds.) | title=A Guide to IUPAC Nomenclature of Organic Compounds 1993 | location=Oxford | publisher=Blackwell Science | year=1994 | isbn = 0-632-03488-2 | url = http://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm}}</ref>An '''isopentyl''' group is a subset of the generic pentyl group. It has the chemical structure -CH<sub>2</sub>CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>. | |||
| Isopentane is commonly used in conjunction with ] to achieve a liquid bath temperature of −160 °C. ] typically contains 1% or less isopentane,<ref>Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. {{doi|10.1002/14356007.a17_073.pub2}}</ref> but it is a significant component of ].<ref name=aver1958>Ivan F. Avery, L. V. Harvey (1958): '''', Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.</ref> | |||
| ==Isomers== | |||
| Isopentane is one of three ] with the ] C<sub>5</sub>H<sub>12</sub>, the others being ] (''n''-pentane) and ] (dimethyl propane). | |||
| ==Nomenclature== | |||
| The traditional name isopentane was still retained in the 1993 ] recommendations,<ref></ref><ref name="panico">{{cite book | editor=Panico, R. | editor2= Powell, W. H. | name-list-style= amp | title=A Guide to IUPAC Nomenclature of Organic Compounds 1993 | location=Oxford | publisher=Blackwell Science | year=1994 | isbn=0-632-03488-2}} | |||
| </ref> but is no longer recommended according to the 2013 recommendations.<ref name="IUPAC2013_652"/> The preferred IUPAC name is the systematic name 2-methylbutane. An '''isopentyl''' group is a subset of the generic pentyl group. It has the chemical structure -CH<sub>3</sub>CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>. | |||
| ==Uses== | ==Uses== | ||
| Isopentane is used in a closed loop in geothermal power production to drive turbines.<ref>Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. | |||
| Isopentane is one of the ingredients in both ]® and ]®.<ref></ref> | |||
| {{Webarchive|url=https://web.archive.org/web/20141018154915/http://hsorka.is/english/HSProduction/Svartsengi/OrkuverIV.aspx |date=2014-10-18 }}</ref> | |||
| Isopentane is used, in conjunction with ] or liquid nitrogen, to freeze ] for ]ing in ]. | |||
| <ref>{{Cite web|url=http://www.uab.edu/research/administration/offices/ARP/ComparativePathology/Pathology/Histopathology/TissueSubmission/Pages/Freezing-Tissues-for-Cryosectioning.aspx|title = Animal Resources Program - the Office of the Vice President for Research | UAB}}</ref> | |||
| Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common ]-derived ] that is condensed from natural gas.<ref name=aver1958/> It has a substantially higher ] (RON 93.7) than ''n''-pentane (61.7), and therefore there is interest in conversion from the latter.<ref name=wang2014>Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". ''International Journal of Thermophysics'', volume 35, pages 1450–1464. {{doi|10.1007/s10765-014-1718-x}}</ref> | |||
| ==References== | ==References== | ||
| Line 95: | Line 101: | ||
| * | * | ||
| * (online version of the "''Blue Book''") | * (online version of the "''Blue Book''") | ||
| {{Alkanes}} | {{Alkanes}} | ||
| {{Authority control}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 13:57, 9 April 2024
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methylbutane | |
| Other names Isopentane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1730723 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.039 |
| EC Number |
|
| Gmelin Reference | 49318 |
| MeSH | isopentane |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1265 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H12 |
| Molar mass | 72.151 g·mol |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 616 mg mL |
| Melting point | −161 to −159 °C; −258 to −254 °F; 112 to 114 K |
| Boiling point | 27.8 to 28.2 °C; 81.9 to 82.7 °F; 300.9 to 301.3 K |
| Vapor pressure | 76.992 kPa (at 20 °C) |
| Henry's law constant (kH) |
7.2 nmol Pa kg |
| UV-vis (λmax) | 192 nm |
| Refractive index (nD) | 1.354 |
| Viscosity | 0.214 cP (at 20 °C) |
| Thermochemistry | |
| Heat capacity (C) | 164.85 J K mol |
| Std molar entropy (S298) |
260.41 J K mol |
| Std enthalpy of formation (ΔfH298) |
−179.1–−177.3 kJ mol |
| Std enthalpy of combustion (ΔcH298) |
~ 3.3 MJ mol, 19,664 Btu/lb |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H224, H301, H302, H305, H336, H411 |
| Precautionary statements | P210, P261, P273, P301+P310, P331 |
| NFPA 704 (fire diamond) |
 |
| Flash point | −51 °C (−60 °F; 222 K) |
| Autoignition temperature |
420 °C (788 °F; 693 K) |
| Explosive limits | 1.4–8.3% |
| Related compounds | |
| Related alkanes | |
| Related compounds | 2-Ethyl-1-butanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane, but it is a significant component of natural gasoline.
Nomenclature
The traditional name isopentane was still retained in the 1993 IUPAC recommendations, but is no longer recommended according to the 2013 recommendations. The preferred IUPAC name is the systematic name 2-methylbutane. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Uses
Isopentane is used in a closed loop in geothermal power production to drive turbines.
Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology.
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common petroleum-derived gasoline that is condensed from natural gas. It has a substantially higher octane rating (RON 93.7) than n-pentane (61.7), and therefore there is interest in conversion from the latter.
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names 'isobutane', 'isopentane' and 'neopentane' are no longer recommended.
- James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 doi:10.1021/ie990588m
- Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_073.pub2
- ^ Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States, Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.
- Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- Panico, R. & Powell, W. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
- Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. HS Orka HF Energy Plant IV Archived 2014-10-18 at the Wayback Machine
- "Animal Resources Program - the Office of the Vice President for Research | UAB".
- Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". International Journal of Thermophysics, volume 35, pages 1450–1464. doi:10.1007/s10765-014-1718-x
External links
- International Chemical Safety Card 1153
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
| Alkanes | |
|---|---|
| |