| Revision as of 01:59, 13 March 2012 editVarlaam (talk | contribs)Extended confirmed users64,824 editsm →Preparation← Previous edit | Latest revision as of 17:14, 2 May 2024 edit undoKeresluna (talk | contribs)Autopatrolled, Extended confirmed users4,082 edits move from chembox | ||
| (42 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | | verifiedrevid = 428906341 | ||
| ⚫ | | |
||
| ⚫ | | Name = Praseodymium(III) chloride | ||
| ⚫ | | ImageFileL1 = UCl3.png | ||
| ⚫ | | ImageFile1 = Praseodymium(III)-chloride-heptahydrate.jpg | ||
| | ImageFile2 = | |||
| ⚫ | | ImageFileL1 = UCl3 without caption.png | ||
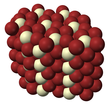
| | ImageFileR1 = Cerium bromide (space filling) 2.png | | ImageFileR1 = Cerium bromide (space filling) 2.png | ||
| | |
| ImageName = | ||
| | |
| IUPACName = Praseodymium(III) chloride | ||
| | |
| OtherNames = Praseodymium chloride; praseodymium trichloride | ||
| | |
|Section1={{Chembox Identifiers | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| CASNo = 10361-79-2 | ||
| ⚫ | | |
||
| ⚫ | | |
||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | UNII = 1JB99PM4G8 | |||
| ⚫ | | RTECS = | ||
| | PubChem = 66317 | |||
| | InChI = 1S/3ClH.Pr/h3*1H;/q;;;+3/p-3 | |||
| | SMILES = Cl(Cl)Cl | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = PrCl<sub>3</sub> | ||
| | |
| MolarMass = 247.24 g/mol (anhydrous)<br/>373.77 g/mol (heptahydrate) | ||
| | |
| Appearance = blue-green solid (anhydrous)<br/>light green solid (heptahydrate) | ||
| | |
| Density = 4.02 g/cm<sup>3</sup> (anhydrous)<br/>2.250 g/cm<sup>3</sup> (heptahydrate) | ||
| | |
| Solubility = 104.0 g/100 ml (13 °C) | ||
| | |
| MeltingPtC = 786 | ||
| | |
| BoilingPtC = 1710 | ||
| | |
| pKa = | ||
| | |
| pKb = | ||
| | MagSus = +44.5·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | |
|Section3={{Chembox Structure | ||
| | |
| Coordination = Tricapped trigonal prismatic<br />(nine-coordinate) | ||
| | |
| CrystalStruct = ] (]), ] | ||
| | |
| SpaceGroup = P6<sub>3</sub>/m, No. 176 | ||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | |
| ExternalSDS = | ||
| ⚫ | | MainHazards = Irritant | ||
| | EUIndex = Not listed | |||
| | FlashPt = | |||
| ⚫ | | |
||
| | |
| HPhrases = | ||
| | |
| PPhrases = | ||
| | |
| GHS_ref = | ||
| }} | |||
| | |
|Section8={{Chembox Related | ||
| | |
| OtherAnions = ], ]<br/>]<br/>] | ||
| | |
| OtherCations = ]<br/>] | ||
| }} | }} | ||
| }} | }} | ||
| '''Praseodymium(III) chloride''' is the ] with the ] ]]<sub>3</sub>. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green hepta]. | '''Praseodymium(III) chloride''' is the ] with the ] ]]<sub>3</sub>. Like other ]s, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green hepta]. | ||
| ==Preparation== | ==Preparation== | ||
| Praseodymium(III) chloride is prepared by treating praseodymium metal |
Praseodymium(III) chloride is prepared by treating praseodymium metal with ]:<ref name=ref7 /><ref name=ref6>{{cite journal|url=https://pubs.acs.org/doi/10.1021/ja01472a010|author1=L.F. Druding|author2= J.D. Corbett|title=Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide|journal=J. Am. Chem. Soc.|volume=83|issue=11|pages=2462-2467|date=1961-06-01|doi=10.1021/ja01472a010}}</ref> | ||
| :2 Pr + 6 HCl → 2 PrCl<sub>3</sub> + 3 H<sub>2</sub> | :2 Pr + 6 HCl → 2 PrCl<sub>3</sub> + 3 H<sub>2</sub> | ||
| It is usually purified by vacuum sublimation.<ref name=ref1 /> | It is usually purified by vacuum sublimation.<ref name=ref1 /> | ||
| Line 53: | Line 66: | ||
| :Pr<sub>2</sub>(CO<sub>3</sub>)<sub>3</sub> + 6 HCl + 15 H<sub>2</sub>O → 2 Cl<sub>3</sub> + 3 CO<sub>2</sub> | :Pr<sub>2</sub>(CO<sub>3</sub>)<sub>3</sub> + 6 HCl + 15 H<sub>2</sub>O → 2 Cl<sub>3</sub> + 3 CO<sub>2</sub> | ||
| Anhydrous PrCl<sub>3</sub> can be made by thermal dehydration of the hydrate at 400 |
PrCl<sub>3</sub>∙7H<sub>2</sub>O is a hygroscopic substance, that will not crystallize from the ] unless it is left to dry in a desiccator. Anhydrous PrCl<sub>3</sub> can be made by thermal dehydration of the hydrate at 400 °C in the presence of ], the so-called ].<ref name=ref1 /><ref name=ref4>{{cite journal|url=https://www.sciencedirect.com/science/article/abs/pii/0022190262800347|author1=M.D. Taylor|author2=P.C. Carter|title= Preparation of anhydrous lanthanide halides, especially iodides|journal=J. Inorg. Nucl. Chem.|volume=24|issue=4|pages=387-391|date=April 1962|doi=10.1016/0022-1902(62)80034-7}}</ref><ref name="Kutscher">{{cite journal|url=https://www.sciencedirect.com/science/article/abs/pii/0020165071802532|author1=J. Kutscher|author2=A. Schneider|title=Notiz zur Präparation von wasserfreien Lanthaniden-Haloge-niden, Insbesondere von Jodiden|journal=Inorg. Nucl. Chem. Lett.|volume=7|issue=9|pages=815-819|date=September 1971|doi=10.1016/0020-1650(71)80253-2|language=de}}</ref> Alternatively the hydrate can be dehydrated using ].<ref name=ref1/><ref name=ref5>{{cite journal|url=https://www.sciencedirect.com/science/article/abs/pii/0022190258800731|author1=J.H. Freeman|author2=M.L. Smith|title=The preparation of anhydrous inorganic chlorides by dehydration with thionyl chloride|journal=J. Inorg. Nucl. Chem.|volume=7|issue=3|pages=224-227|date=October 1958|doi=10.1016/0022-1902(58)80073-1}}</ref> | ||
| ==Reactions== | ==Reactions== | ||
| Praseodymium(III) chloride is ]ic, classified as "hard" according to the ]. |
Praseodymium(III) chloride is ]ic, classified as "hard" according to the ]. Rapid heating of the ] may cause small amounts of ].<ref name=ref1>{{cite book|author1=F.T. Edelmann|author2= P. Poremba|title=Synthetic Methods of Organometallic and Inorganic Chemistry|volume=6|publisher= Georg Thieme Verlag|location=Stuttgart|date= 1997|isbn=978-31-319-3921-0}}</ref> PrCl<sub>3</sub> forms a stable Lewis acid-base complex K<sub>2</sub>PrCl<sub>5</sub> by reaction with ]; this compound shows interesting ] and ] properties.<ref name=ref7>{{cite journal|url=https://www.semanticscholar.org/paper/Spectroscopic-and-magnetic-studies-of-the-ternary-Cybi%C5%84ska-Sokolnicki/2af96eacdd78d2ec04ac3c0342ca6d875f76130e|author1=J. Cybinska|author2= J. Sokolnicki|author3= J. Legendziewicz|author4= G. Meyer|title=Spectroscopic and magnetic studies of the ternary praseodymium chloride K<sub>2</sub>PrCl<sub>5</sub>|journal=Journal of Alloys and Compounds|volume=341|pages=115–123|date=2002-07-17|doi=10.1016/S0925-8388(02)00089-0}}</ref> | ||
| ] solutions of praseodymium(III) chloride can be used to prepare insoluble praseodymium(III) compounds. For example, praseodymium(III) phosphate and praseodymium(III) fluoride can be prepared by reaction with ] and ], respectively: | ] solutions of praseodymium(III) chloride can be used to prepare insoluble praseodymium(III) compounds. For example, praseodymium(III) phosphate and praseodymium(III) fluoride can be prepared by reaction with ] and ], respectively: | ||
| Line 62: | Line 75: | ||
| :PrCl<sub>3</sub> + K<sub>3</sub>PO<sub>4</sub> → PrPO<sub>4</sub> + 3 KCl | :PrCl<sub>3</sub> + K<sub>3</sub>PO<sub>4</sub> → PrPO<sub>4</sub> + 3 KCl | ||
| :PrCl<sub>3</sub> + 3 NaF → PrF<sub>3</sub> + 3 NaCl | :PrCl<sub>3</sub> + 3 NaF → PrF<sub>3</sub> + 3 NaCl | ||
| :2PrCl<sub>3</sub> + 3 Na<sub>2</sub>CO<sub>3</sub>----> Pr<sub>2</sub>CO<sub>3</sub> + 6NaCl | |||
| ⚫ | When heated with alkali metal chlorides, it forms a series of ternary (compounds containing three different elements) materials with the formulae MPr<sub>2</sub>Cl<sub>7</sub>, M<sub>3</sub>PrCl<sub>6</sub>, M<sub>2</sub>PrCl<sub>5</sub>, and M<sub>3</sub>Pr<sub>2</sub>Cl<sub>9</sub> where M = K, Rb, Cs.<ref>{{cite journal|author=Gerd Meyer|title=Ternary Chlorides and Bromides of the Rare-Earth Elements|journal=Inorganic Syntheses|year=1990|volume=30|pages=72–81|doi=10.1002/9780470132616.ch15}}</ref> | ||
| ] | |||
| ⚫ | When heated with alkali metal chlorides, it forms a series of ternary (compounds containing three different elements) materials with the formulae MPr<sub>2</sub>Cl<sub>7</sub>, M<sub>3</sub>PrCl<sub>6</sub>, M<sub>2</sub>PrCl<sub>5</sub>, and M<sub>3</sub>Pr<sub>2</sub>Cl<sub>9</sub> where M = K, Rb, Cs.<ref>Gerd Meyer |
||
| ==References== | ==References== | ||
| Line 71: | Line 86: | ||
| #<!--old 2--> ''CRC Handbook of Chemistry and Physics'' (58th edition), CRC Press, West Palm Beach, Florida, 1977. | #<!--old 2--> ''CRC Handbook of Chemistry and Physics'' (58th edition), CRC Press, West Palm Beach, Florida, 1977. | ||
| #<!--old 3--> N. N. Greenwood, A. Earnshaw, ''Chemistry of the Elements'', Pergamon Press, 1984. | #<!--old 3--> N. N. Greenwood, A. Earnshaw, ''Chemistry of the Elements'', Pergamon Press, 1984. | ||
| #<!--old 8--> S. Sugiyama, T. Miyamoto, H. Hayashi, M. Tanaka, J. B. |
#<!--old 8--> S. Sugiyama, T. Miyamoto, H. Hayashi, M. Tanaka, J. B. Moffatt, "Effects of chlorine additives in the gas- and solid-phases on the oxidative dehydrogenation of ethane over praseodymium oxide", ''Journal of Molecular Catalysis A'', '''118''', 129-136 (1997). | ||
| #<!--old 9--> {{cite journal | #<!--old 9--> {{cite journal | ||
| | title = Rare Earth Metal-Metal Halide Systems. VI. Praseodymium Chloride | | title = Rare Earth Metal-Metal Halide Systems. VI. Praseodymium Chloride | ||
| | |
|author1=Druding L. F. |author2=Corbett J. D. |author3=Ramsey B. N. | journal = ] | ||
| | journal = ] | |||
| | year = 1963 | | year = 1963 | ||
| | volume = 2 | | volume = 2 | ||
| Line 84: | Line 98: | ||
| {{Praseodymium compounds}} | {{Praseodymium compounds}} | ||
| {{Chlorides}} | |||
| {{Lanthanide halides}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 17:14, 2 May 2024
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Praseodymium(III) chloride | |||
| Other names Praseodymium chloride; praseodymium trichloride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ECHA InfoCard | 100.030.710 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | PrCl3 | ||
| Molar mass | 247.24 g/mol (anhydrous) 373.77 g/mol (heptahydrate) | ||
| Appearance | blue-green solid (anhydrous) light green solid (heptahydrate) | ||
| Density | 4.02 g/cm (anhydrous) 2.250 g/cm (heptahydrate) | ||
| Melting point | 786 °C (1,447 °F; 1,059 K) | ||
| Boiling point | 1,710 °C (3,110 °F; 1,980 K) | ||
| Solubility in water | 104.0 g/100 ml (13 °C) | ||
| Magnetic susceptibility (χ) | +44.5·10 cm/mol | ||
| Structure | |||
| Crystal structure | hexagonal (UCl3 type), hP8 | ||
| Space group | P63/m, No. 176 | ||
| Coordination geometry | Tricapped trigonal prismatic (nine-coordinate) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Irritant | ||
| Related compounds | |||
| Other anions | Praseodymium(III) oxide, Praseodymium(III) fluoride Praseodymium bromide praseodymium iodide | ||
| Other cations | Cerium(III) chloride Neodymium(III) chloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.
Preparation
Praseodymium(III) chloride is prepared by treating praseodymium metal with hydrogen chloride:
- 2 Pr + 6 HCl → 2 PrCl3 + 3 H2
It is usually purified by vacuum sublimation.
Hydrated salts of praseodymium(III) chloride can be prepared by treatment of either praseodymium metal or praseodymium(III) carbonate with hydrochloric acid:
- Pr2(CO3)3 + 6 HCl + 15 H2O → 2 Cl3 + 3 CO2
PrCl3∙7H2O is a hygroscopic substance, that will not crystallize from the mother liquor unless it is left to dry in a desiccator. Anhydrous PrCl3 can be made by thermal dehydration of the hydrate at 400 °C in the presence of ammonium chloride, the so-called ammonium chloride route. Alternatively the hydrate can be dehydrated using thionyl chloride.
Reactions
Praseodymium(III) chloride is Lewis acidic, classified as "hard" according to the HSAB concept. Rapid heating of the hydrate may cause small amounts of hydrolysis. PrCl3 forms a stable Lewis acid-base complex K2PrCl5 by reaction with potassium chloride; this compound shows interesting optical and magnetic properties.
Aqueous solutions of praseodymium(III) chloride can be used to prepare insoluble praseodymium(III) compounds. For example, praseodymium(III) phosphate and praseodymium(III) fluoride can be prepared by reaction with potassium phosphate and sodium fluoride, respectively:
- PrCl3 + K3PO4 → PrPO4 + 3 KCl
- PrCl3 + 3 NaF → PrF3 + 3 NaCl
- 2PrCl3 + 3 Na2CO3----> Pr2CO3 + 6NaCl
When heated with alkali metal chlorides, it forms a series of ternary (compounds containing three different elements) materials with the formulae MPr2Cl7, M3PrCl6, M2PrCl5, and M3Pr2Cl9 where M = K, Rb, Cs.

References
- ^ J. Cybinska; J. Sokolnicki; J. Legendziewicz; G. Meyer (2002-07-17). "Spectroscopic and magnetic studies of the ternary praseodymium chloride K2PrCl5". Journal of Alloys and Compounds. 341: 115–123. doi:10.1016/S0925-8388(02)00089-0.
- L.F. Druding; J.D. Corbett (1961-06-01). "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide". J. Am. Chem. Soc. 83 (11): 2462–2467. doi:10.1021/ja01472a010.
- ^ F.T. Edelmann; P. Poremba (1997). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. 6. Stuttgart: Georg Thieme Verlag. ISBN 978-31-319-3921-0.
- M.D. Taylor; P.C. Carter (April 1962). "Preparation of anhydrous lanthanide halides, especially iodides". J. Inorg. Nucl. Chem. 24 (4): 387–391. doi:10.1016/0022-1902(62)80034-7.
- J. Kutscher; A. Schneider (September 1971). "Notiz zur Präparation von wasserfreien Lanthaniden-Haloge-niden, Insbesondere von Jodiden". Inorg. Nucl. Chem. Lett. (in German). 7 (9): 815–819. doi:10.1016/0020-1650(71)80253-2.
- J.H. Freeman; M.L. Smith (October 1958). "The preparation of anhydrous inorganic chlorides by dehydration with thionyl chloride". J. Inorg. Nucl. Chem. 7 (3): 224–227. doi:10.1016/0022-1902(58)80073-1.
- Gerd Meyer (1990). "Ternary Chlorides and Bromides of the Rare-Earth Elements". Inorganic Syntheses. 30: 72–81. doi:10.1002/9780470132616.ch15.
Further reading
- CRC Handbook of Chemistry and Physics (58th edition), CRC Press, West Palm Beach, Florida, 1977.
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, 1984.
- S. Sugiyama, T. Miyamoto, H. Hayashi, M. Tanaka, J. B. Moffatt, "Effects of chlorine additives in the gas- and solid-phases on the oxidative dehydrogenation of ethane over praseodymium oxide", Journal of Molecular Catalysis A, 118, 129-136 (1997).
- Druding L. F.; Corbett J. D.; Ramsey B. N. (1963). "Rare Earth Metal-Metal Halide Systems. VI. Praseodymium Chloride". Inorganic Chemistry. 2 (4): 869–871. doi:10.1021/ic50008a055.
| Praseodymium compounds | |||
|---|---|---|---|
| Pr(II) | |||
| Pr(III) |
| ||
| Pr(III,IV) | |||
| Pr(IV) | |||
| Pr(V) | |||
| Lanthanide salts of halides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||