| Revision as of 00:24, 6 April 2012 editRezabot (talk | contribs)Extended confirmed users48,194 editsm r2.7.1) (Robot: Adding fa:ایبیسیان← Previous edit | Latest revision as of 10:56, 13 September 2024 edit undoTom.Reding (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, Template editors3,879,760 editsm WP:STUBSPACING followupTag: AWB | ||
| (28 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
| {{For|Indian IT Company Abcence|Abcence}} | |||
| {{ |
{{For|the Australian TV station|ABN (TV station)}} | ||
| {{Unreferenced|date =September 2007}} | |||
| {{Chembox | {{Chembox | ||
| | |
|Verifiedfields = changed | ||
| |Watchedfields = changed | |||
| | |
|verifiedrevid = 477235009 | ||
| | |
|ImageFile1 = ABCN-2D-skeletal.png | ||
| | |
|ImageFile1_Ref = {{chemboximage|correct|??}} | ||
| | ImageSize1 = | |||
| | |
|ImageName1 = Stereo, skeletal formula of (''Z'')-ABCN | ||
| | |
|ImageFile2 = ABCN-3D-sticks.png | ||
| | |
|ImageFile2_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | |ImageName2 = Stick model of (''Z'')-ABCN | ||
| | ImageSize2 = | |||
| ⚫ | |ImageFile3 = ABCN-3D-vdW.png | ||
| ⚫ | | |
||
| ⚫ | |ImageFile3_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | | |
||
| ⚫ | |ImageName3 = Spacefill model of (''Z'')-ABCN | ||
| ⚫ | | |
||
| ⚫ | |SystematicName = 1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | ||
| | ImageSize3 = | |||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | |
||
| ⚫ | |Abbreviations = ACHN | ||
| ⚫ | | |
||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| ⚫ | | |
||
| ⚫ | |CASNo = 2094-98-6 | ||
| ⚫ | | |
||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| |UNII = 9Y0B93KKUS | |||
| | |
|PubChem = 74978 | ||
| | PubChem_Ref = {{Pubchemcite|correct|pubchem}} | |||
| | |
|ChemSpiderID = 21159585 | ||
| | |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID2 = 21427655 | ||
| | |
|ChemSpiderID2_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID2_Comment = <small>(''Z'')</small> | ||
| | |
|ChemSpiderID1 = 67533 | ||
| | |
|ChemSpiderID1_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID1_Comment = <small>(''E'')</small> | ||
| | |
|EINECS = 218-254-8 | ||
| | |
|UNNumber = 3226 | ||
| | |
|Beilstein = 960744 | ||
| | |
|SMILES = N#CC1(CCCCC1)N=NC1(CCCCC1)C#N | ||
| | |
|StdInChI = 1S/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2 | ||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|InChI = 1/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2 | ||
| | |
|StdInChIKey = KYIKRXIYLAGAKQ-UHFFFAOYSA-N | ||
| | |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|InChIKey = KYIKRXIYLAGAKQ-UHFFFAOYAM | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| |C=14 | H=20 | N=4 | |||
| | Formula = {{Chem|C|14|N|4|H|20}} | |||
| |MeltingPtC = 114 to 118<ref name=SA> at ]</ref> | |||
| | MolarMass = 244.3354 g mol<sup>−1</sup> | |||
| |MeltingPt_notes = decomposes near 80 °C | |||
| | ExactMass = 244.168796660 g mol<sup>−1</sup> | |||
| | MeltingPtCL = 114 | |||
| | MeltingPtCH = 118 | |||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | |
|GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} | ||
| | |
|GHSSignalWord = Danger | ||
| | |
|HPhrases = {{H-phrases|242|315|319|335}} | ||
| | |
|PPhrases = {{P-phrases|261|305+351+338}} | ||
| | EUClass = {{Hazchem F}} {{Hazchem Xi}} | |||
| | RPhrases = {{R11}}, {{R36/37/38}} | |||
| | SPhrases = {{S26}} | |||
| }} | }} | ||
| }} | }} | ||
| '''1, |
'''1,1′-Azobis(cyclohexanecarbonitrile)''' or '''ACHN''' is a ].<ref name=SA/> The molecular formula is NCC<sub>6</sub>H<sub>10</sub>N=NC<sub>6</sub>H<sub>10</sub>CN. It is a white solid that is soluble in aromatic solvents.<ref>{{cite journal|title=1,1'-Azobis-1-cyclohexanenitrile|author=Steven A. Kates, Fernando Albericio | ||
| |journal=E-EROS Encyclopedia of Reagents for Organic Synthesis|year=2001|doi=10.1002/047084289X.ra120}}</ref> | |||
| NCC<sub>6</sub>H<sub>10</sub>N=NC<sub>6</sub>H<sub>10</sub>CN. It is classified as highly flammable and an irritant. | |||
| ACHN has a 10-hour ] in ] at 88 °C.<ref name=SA/> | |||
| ==See also== | ==See also== | ||
| * ] (AIBN) is another commonly used free radical initiator | * ] (AIBN) is another commonly used free radical initiator | ||
| ==References== | |||
| ⚫ | {{Organic-compound-stub}} | ||
| {{reflist}} | |||
| ] | ] | ||
| Line 73: | Line 69: | ||
| ] | ] | ||
| ] | |||
| ⚫ | {{Organic-compound-stub}} | ||
| ] | |||
Latest revision as of 10:56, 13 September 2024
For Indian IT Company Abcence, see Abcence. For the Australian TV station, see ABN (TV station).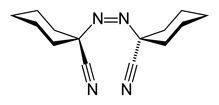
| |
| |

| |
| Names | |
|---|---|
| Systematic IUPAC name 1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | ACHN |
| Beilstein Reference | 960744 |
| ChemSpider | |
| ECHA InfoCard | 100.016.595 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 3226 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H20N4 |
| Molar mass | 244.342 g·mol |
| Melting point | 114 to 118 °C (237 to 244 °F; 387 to 391 K) decomposes near 80 °C |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H242, H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
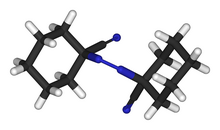
1,1′-Azobis(cyclohexanecarbonitrile) or ACHN is a radical initiator. The molecular formula is NCC6H10N=NC6H10CN. It is a white solid that is soluble in aromatic solvents.
ACHN has a 10-hour half-life in toluene at 88 °C.
See also
- Azobisisobutylonitrile (AIBN) is another commonly used free radical initiator
References
- ^ 1,1′-Azobis(cyclohexanecarbonitrile) at Sigma-Aldrich
- Steven A. Kates, Fernando Albericio (2001). "1,1'-Azobis-1-cyclohexanenitrile". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra120.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |