| Revision as of 03:21, 15 July 2012 editFleecemaster (talk | contribs)9 edits →Stability of soap films← Previous edit | Latest revision as of 11:44, 25 December 2024 edit undoToadspike (talk | contribs)Extended confirmed users, Page movers, New page reviewers, Pending changes reviewers10,044 editsm →Stability: nom nom...yummy sandwichTag: Visual edit | ||
| (48 intermediate revisions by 31 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Thin layers of liquid surrounded by air}} | |||
| ⚫ | '''Soap films''' are thin layers of ] (usually water-based) surrounded by air. For example, if two ]s come |
||
| {{Multiple issues|section=no| | |||
| {{technical|date=June 2020}} | |||
| {{More sources needed|date=June 2020}} | |||
| {{lead extra info|date=June 2020}} | |||
| {{Missing information|governing physics, links with adsorption and nucleation, relevance in science and daily life|date=June 2020}} | |||
| }} | |||
| {{distinguish|Soap opera}} | |||
| ⚫ | == Stability |
||
| ⚫ | '''Soap films''' are thin layers of ] (usually water-based) surrounded by air. For example, if two ]s come into contact, they merge and a thin film is created in between. Thus, ]s are composed of a network of films connected by ]. Soap films can be used as model systems for minimal surfaces, which are widely used in mathematics. | ||
| ⚫ | Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of ], is necessary to stabilize the film. Most of the time, surfactants are ], which means they are |
||
| ⚫ | == Stability == | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | Daily experience{{Citation needed|date=October 2020|reason=Who's daily experience?}} shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of ], is necessary to stabilize the film. Most of the time, surfactants are ], which means they are molecules with both a ] and a ] part. Thus, they are arranged preferentially at the air/water interface (see figure 1). | ||
| ⚫ | Surfactants stabilize films because they create a repulsion between both surfaces of the film, preventing it from thinning and consequentially bursting. This can be shown quantitatively through calculations relating to ]. The main repulsion mechanisms are steric (the surfactants can not interlace) and electrostatic (if surfactants are charged). | ||
| ⚫ | Surfactants stabilize films because they create a repulsion between both surfaces of the film, preventing it from thinning and consequentially bursting. This can be shown quantitatively through calculations relating to ]. The main repulsion mechanisms are ] (the surfactants can not interlace) and electrostatic (if surfactants are charged). | ||
| ⚫ | Moreover, surfactants make the film more stable toward thickness fluctuations due to the ]. This gives some elasticity to the interface: if surface |
||
| ⚫ | Moreover, surfactants make the film more stable toward thickness fluctuations due to the ]. This gives some elasticity to the interface: if surface concentrations are not homogeneously dispersed at the surface, Marangoni forces will tend to re-homogenize the surface concentration (see figure 2). | ||
| ⚫ | Even in presence of stabilizing surfactants, a soap film does not last forever. Water evaporates with time |
||
| ⚫ | Even in the presence of stabilizing surfactants, a soap film does not last forever. Water evaporates with time depending on the humidity of the atmosphere. Moreover, as soon as a film is not perfectly horizontal, the liquid flows toward the bottom due to gravity and the liquid accumulates at the bottom. At the top, the film thins and bursts. | ||
| == Importance of surface tension: minimal surfaces == | == Importance of surface tension: minimal surfaces == | ||
| From a mathematical point of view, soap films are ]s. ] is the energy that is required to produce the surface, per unit area. A film—like any body or structure—prefers to exist in ]. In order to minimize its energy, a droplet of liquid in free space naturally assumes a spherical shape, which has the minimum surface area for a given volume. ]s and films can exist in of the presence of other forces, like ] and the ] to the atoms of a substrate. The latter phenomenon is called ]: binding forces between the substrate atoms and the film atoms can cause the total energy to decrease. In that case, the lowest energy configuration for the body would be one where as many film atoms as possible are as close as possible to the substrate. That would result in an infinitely thin film, infinitely widely spread out over the substrate. In reality, the effect of adherent wetting (causing surface maximization) and the effect of surface tension (causing surface minimization) would balance each other out: the stable configuration can be a droplet, a puddle, or a thin film, depending on the forces that work on the body.<ref>{{Cite book|last=Gennes, Pierre-Gilles de.|title=Capillarity and wetting phenomena : drops, bubbles, pearls, waves|date=2004|publisher=Springer|others=Brochard-Wyart, Françoise., Quéré, David.|isbn=0-387-00592-7|location=New York|oclc=51559047}}</ref> | |||
| From a mathematical point of view, soap films are considered as minimal surfaces. See for instance the approximate numerical method for minimal surfaces form finding at ]. ], which measures the energy needed to create a surface indeed acts as a physical surface minimizer: since energy is proportional to the soap film surface, the film deforms to minimize its surface and, thus its energy. | |||
| ⚫ | == Colours == | ||
| Depending on the support of the soap film, the latter then naturally takes the minimal surface. On a flat support, the soap film is usually flat. But, on more complicate support, it takes the minimal achievable surface. | |||
| ⚫ | ] | ||
| ⚫ | == Colours |
||
| ⚫ | The ] colours of a soap film are caused by ] of (internally and externally) reflected light waves, a process called ] and are determined by the thickness of the film. This phenomenon is not the same as the origin of ] colours (caused by the ] of internally reflected light), but rather is the same as the phenomenon causing the colours in an oil slick on a wet road. | ||
| ⚫ | ] | ||
| ⚫ | ==Drainage== | ||
| ⚫ | The ] |
||
| ⚫ | ] | ||
| ⚫ | ==Drainage |
||
| If |
If ]s are well chosen<ref name="Ball61">Ball, 2009. pp. 61–67</ref> and the atmospheric humidity and air movements are suitably controlled, a horizontal soap film can last from minutes to hours. In contrast, a vertical soap film is affected by gravity and so the liquid tends to drain, causing the soap film to thin at the top. Colour depends on film thickness, which accounts for the coloured interference fringes that can be seen at the top of figure 4. | ||
| ==Black spots== | |||
| ⚫ | ] | ||
| ] | |||
| ⚫ | ==Bursting |
||
| During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:<ref name="Pugh2016">{{cite book|last1=Pugh|first1=Robert J.|title=Bubble and Foam Chemistry|chapter=Soap bubbles and thin films|year=2016|pages=84–111|publisher=Cambridge|doi=10.1017/CBO9781316106938.004|isbn=9781316106938}}</ref> | |||
| * Common black films, around 50 nm in thickness, and | |||
| * Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid. | |||
| As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes. | |||
| ⚫ | If a soap film is unstable, it ends by bursting. A hole is created somewhere in the film and opens very rapidly. |
||
| ⚫ | ==Bursting== | ||
| ⚫ | If a soap film is unstable, it ends by bursting. A hole is created somewhere in the film and opens very rapidly. Surface tension indeed leads to surface minimization and, thus, to film disappearance. The hole aperture is not instantaneous and is slowed by the liquid inertia. The balance between the forces of inertia and surface tension leads to the opening velocity:<ref>{{cite journal | last=Culick | first=F. E. C. | title=Comments on a Ruptured Soap Film | journal=Journal of Applied Physics | publisher=AIP Publishing | volume=31 | issue=6 | year=1960 | issn=0021-8979 | doi=10.1063/1.1735765 | pages=1128–1129| bibcode=1960JAP....31.1128C | url=https://authors.library.caltech.edu/10315/1/CULjap60.pdf }}</ref> | ||
| <math>V=\sqrt{\frac{2\gamma}{\rho h}}</math> where <math>\gamma</math> is the liquid surface tension, <math>\rho</math> is the liquid density and <math>h</math> is the film thickness. | <math>V=\sqrt{\frac{2\gamma}{\rho h}}</math> where <math>\gamma</math> is the liquid surface tension, <math>\rho</math> is the liquid density and <math>h</math> is the film thickness. | ||
| ==Fun== | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| ===General sources=== | |||
| Giant film | |||
| * {{cite book | title=Shapes. Nature's Patterns: a tapestry in three parts | url=https://archive.org/details/shapesnaturespat00ball_515 | url-access=limited | publisher=Oxford University Press | author=Ball, Philip | year=2009 | pages=–67, 81–97, 291–292 | isbn=978-0-19-960486-9}} | |||
| {{Foam scales and properties}} | {{Foam scales and properties}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 11:44, 25 December 2024
Thin layers of liquid surrounded by airThis section has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Soap films are thin layers of liquid (usually water-based) surrounded by air. For example, if two soap bubbles come into contact, they merge and a thin film is created in between. Thus, foams are composed of a network of films connected by Plateau borders. Soap films can be used as model systems for minimal surfaces, which are widely used in mathematics.
Stability


Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of surfactants, is necessary to stabilize the film. Most of the time, surfactants are amphiphilic, which means they are molecules with both a hydrophobic and a hydrophilic part. Thus, they are arranged preferentially at the air/water interface (see figure 1).
Surfactants stabilize films because they create a repulsion between both surfaces of the film, preventing it from thinning and consequentially bursting. This can be shown quantitatively through calculations relating to disjoining pressure. The main repulsion mechanisms are steric (the surfactants can not interlace) and electrostatic (if surfactants are charged).
Moreover, surfactants make the film more stable toward thickness fluctuations due to the Marangoni effect. This gives some elasticity to the interface: if surface concentrations are not homogeneously dispersed at the surface, Marangoni forces will tend to re-homogenize the surface concentration (see figure 2).
Even in the presence of stabilizing surfactants, a soap film does not last forever. Water evaporates with time depending on the humidity of the atmosphere. Moreover, as soon as a film is not perfectly horizontal, the liquid flows toward the bottom due to gravity and the liquid accumulates at the bottom. At the top, the film thins and bursts.
Importance of surface tension: minimal surfaces
From a mathematical point of view, soap films are minimal surfaces. Surface tension is the energy that is required to produce the surface, per unit area. A film—like any body or structure—prefers to exist in a state of minimum potential energy. In order to minimize its energy, a droplet of liquid in free space naturally assumes a spherical shape, which has the minimum surface area for a given volume. Puddles and films can exist in of the presence of other forces, like gravity and the intermolecular attraction to the atoms of a substrate. The latter phenomenon is called wetting: binding forces between the substrate atoms and the film atoms can cause the total energy to decrease. In that case, the lowest energy configuration for the body would be one where as many film atoms as possible are as close as possible to the substrate. That would result in an infinitely thin film, infinitely widely spread out over the substrate. In reality, the effect of adherent wetting (causing surface maximization) and the effect of surface tension (causing surface minimization) would balance each other out: the stable configuration can be a droplet, a puddle, or a thin film, depending on the forces that work on the body.
Colours

The iridescent colours of a soap film are caused by interfering of (internally and externally) reflected light waves, a process called thin film interference and are determined by the thickness of the film. This phenomenon is not the same as the origin of rainbow colours (caused by the refraction of internally reflected light), but rather is the same as the phenomenon causing the colours in an oil slick on a wet road.
Drainage
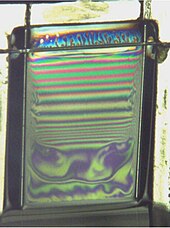
If surfactants are well chosen and the atmospheric humidity and air movements are suitably controlled, a horizontal soap film can last from minutes to hours. In contrast, a vertical soap film is affected by gravity and so the liquid tends to drain, causing the soap film to thin at the top. Colour depends on film thickness, which accounts for the coloured interference fringes that can be seen at the top of figure 4.
Black spots

During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:
- Common black films, around 50 nm in thickness, and
- Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid.
As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes.
Bursting
If a soap film is unstable, it ends by bursting. A hole is created somewhere in the film and opens very rapidly. Surface tension indeed leads to surface minimization and, thus, to film disappearance. The hole aperture is not instantaneous and is slowed by the liquid inertia. The balance between the forces of inertia and surface tension leads to the opening velocity: where is the liquid surface tension, is the liquid density and is the film thickness.
References
- Gennes, Pierre-Gilles de. (2004). Capillarity and wetting phenomena : drops, bubbles, pearls, waves. Brochard-Wyart, Françoise., Quéré, David. New York: Springer. ISBN 0-387-00592-7. OCLC 51559047.
- Ball, 2009. pp. 61–67
- Pugh, Robert J. (2016). "Soap bubbles and thin films". Bubble and Foam Chemistry. Cambridge. pp. 84–111. doi:10.1017/CBO9781316106938.004. ISBN 9781316106938.
- Culick, F. E. C. (1960). "Comments on a Ruptured Soap Film" (PDF). Journal of Applied Physics. 31 (6). AIP Publishing: 1128–1129. Bibcode:1960JAP....31.1128C. doi:10.1063/1.1735765. ISSN 0021-8979.
General sources
- Ball, Philip (2009). Shapes. Nature's Patterns: a tapestry in three parts. Oxford University Press. pp. 61–67, 81–97, 291–292. ISBN 978-0-19-960486-9.
| Foam scales and properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 where
where  is the liquid surface tension,
is the liquid surface tension,  is the liquid density and
is the liquid density and  is the film thickness.
is the film thickness.