| Revision as of 13:07, 27 February 2013 editAddbot (talk | contribs)Bots2,838,809 editsm Bot: Migrating 5 interwiki links, now provided by Wikidata on d:q416114 (Report Errors)← Previous edit | Latest revision as of 17:50, 2 April 2024 edit undoMarbletan (talk | contribs)Extended confirmed users5,666 editsNo edit summary | ||
| (29 intermediate revisions by 31 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Unreferenced stub|auto=yes|date=December 2009}} | |||
| {{Chembox | {{Chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 470602523 | ||
| | ImageFile = Terpinen-4-ol.svg | | ImageFile = Terpinen-4-ol.svg | ||
| | ImageSize = 100px | | ImageSize = 100px | ||
| | PIN = 4-Methyl-1-(propan-2-yl)cyclohex-3-en-1-ol | |||
| | IUPACName = | |||
| | OtherNames = | | OtherNames = | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 10756 | | ChemSpiderID = 10756 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| Line 21: | Line 21: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 562-74-3 | | CASNo = 562-74-3 | ||
| | |
| PubChem = 11230 | ||
| | |
| SMILES = CC1=CCC(CC1)(C(C)C)O | ||
| | |
| MeSHName = terpinenol-4 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=10 | H=18 | O=1 | ||
| | |
| Appearance = | ||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | Autoignition = | |||
| }} | }} | ||
| }} | }} | ||
| '''Terpinen-4-ol''' is an isomer of ] with the chemical formula C<sub>10</sub>H<sub>18</sub>O. A primary constituent of ],<ref name="lact">{{cite web |title=Tea tree oil |url=https://www.ncbi.nlm.nih.gov/books/NBK501884/ |publisher=Drugs and Lactation Database (LactMed), National Library of Medicine, US National Institutes of Health |accessdate=31 July 2019 |date=3 December 2018}}</ref> it is obtained as an ] from the leaves, branches, and bark of '']'' Cheel.<ref name="drugs">{{cite web |title=Tea tree oil |url=https://www.drugs.com/npp/tea-tree-oil.html |publisher=Drugs.com |accessdate=31 July 2019 |date=17 June 2019}}</ref><ref name="Groot">{{cite journal | last1=de Groot | first1=Anton C. | last2=Schmidt | first2=Erich | title=Tea tree oil: contact allergy and chemical composition | journal=Contact Dermatitis | volume=75 | issue=3 | date=13 May 2016 | issn=0105-1873 | doi=10.1111/cod.12591 | pages=129–143| pmid=27173437 | doi-access=free }}</ref><ref>{{ cite journal | author = Hammer, K. A.; Carson, C. F.; Riley, T. V. | title = Effects of ''Melaleuca alternifolia'' (Tea Tree) Essential Oil and the Major Monoterpene Component Terpinen-4-ol on the Development of Single- and Multistep Antibiotic Resistance and Antimicrobial Susceptibility | journal = Antimicrobial Agents and Chemotherapy | year = 2012 | volume = 56 | issue = 2 | pages = 909–915 | doi = 10.1128/AAC.05741-11 | pmid = 22083482 | pmc = 3264233 }}</ref> Despite considerable ] and preliminary ] of terpinen-4-ol and tea tree oil, its biological properties and potential for clinical uses have not been established as of 2019.<ref name=drugs/> It may be a factor in the ] of tea tree oil when used ].<ref name=drugs/><ref name=Groot/> | |||
| '''Terpinen-4-ol''' is a ] with a molecular weight of 154.249. | |||
| Terpinen-4-ol occurs in '']'' and is thought to be the reason why this wood is highly resistant to ]. {{citation needed|date=July 2019}} | |||
| It is considered the primary active ingredient of ]. It is also the compound of highest concentration in the ] of ]. | |||
| ==Synthesis== | |||
| ==Additional images== | |||
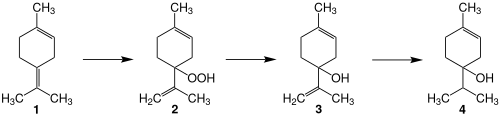
| Terpinen-4-ol can be synthesized from ] ('''1''') by ], reduction of the resulting hydroperoxide ('''2'''), and selective ] of the terminal double bond in '''3'''. | |||
| ⚫ | ] | ||
| ⚫ | :]{{clear-left}} | ||
| {{DEFAULTSORT:Terpinen-4-Ol}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
| ==References== | |||
| {{alcohol-stub}} | |||
| {{reflist}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
Latest revision as of 17:50, 2 April 2024
 | |
| Names | |
|---|---|
| Preferred IUPAC name 4-Methyl-1-(propan-2-yl)cyclohex-3-en-1-ol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.396 |
| MeSH | terpinenol-4 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H18O |
| Molar mass | 154.253 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Terpinen-4-ol is an isomer of terpineol with the chemical formula C10H18O. A primary constituent of tea tree oil, it is obtained as an extract from the leaves, branches, and bark of Melaleuca alternifolia Cheel. Despite considerable basic and preliminary clinical research of terpinen-4-ol and tea tree oil, its biological properties and potential for clinical uses have not been established as of 2019. It may be a factor in the contact dermatitis of tea tree oil when used topically.
Terpinen-4-ol occurs in Juniperus communis and is thought to be the reason why this wood is highly resistant to rot.
Synthesis
Terpinen-4-ol can be synthesized from terpinolene (1) by photooxidation, reduction of the resulting hydroperoxide (2), and selective hydrogenation of the terminal double bond in 3.
References
- "Tea tree oil". Drugs and Lactation Database (LactMed), National Library of Medicine, US National Institutes of Health. 3 December 2018. Retrieved 31 July 2019.
- ^ "Tea tree oil". Drugs.com. 17 June 2019. Retrieved 31 July 2019.
- ^ de Groot, Anton C.; Schmidt, Erich (13 May 2016). "Tea tree oil: contact allergy and chemical composition". Contact Dermatitis. 75 (3): 129–143. doi:10.1111/cod.12591. ISSN 0105-1873. PMID 27173437.
- Hammer, K. A.; Carson, C. F.; Riley, T. V. (2012). "Effects of Melaleuca alternifolia (Tea Tree) Essential Oil and the Major Monoterpene Component Terpinen-4-ol on the Development of Single- and Multistep Antibiotic Resistance and Antimicrobial Susceptibility". Antimicrobial Agents and Chemotherapy. 56 (2): 909–915. doi:10.1128/AAC.05741-11. PMC 3264233. PMID 22083482.
{{cite journal}}: CS1 maint: multiple names: authors list (link)