| Revision as of 22:04, 16 December 2013 editDePiep (talk | contribs)Extended confirmed users294,285 edits format temperatures Flashpoint, Autoignition, replaced: FlashPt → FlashPtC using AWB← Previous edit | Latest revision as of 10:04, 13 January 2024 edit undoMaxim Masiutin (talk | contribs)Extended confirmed users, IP block exemptions, Pending changes reviewers31,057 edits Added isbn. | ||
| (19 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Intermediate Chemical in H2O2 Synthesis}} | |||
| {{chembox | |||
| {{Chembox | |||
| | |
| Verifiedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 477213314 | ||
| | Name = 2-Ethylanthraquinone | | Name = 2-Ethylanthraquinone | ||
| | ImageFile = 2-Ethylanthraquinone.svg | | ImageFile = 2-Ethylanthraquinone.svg | ||
| Line 9: | Line 10: | ||
| | ImageName1 = Ball-and-stick model | | ImageName1 = Ball-and-stick model | ||
| | ImageSize1 = 230px | | ImageSize1 = 230px | ||
| | PIN = 2-Ethylanthracene-9,10-dione | |||
| | OtherNames = 2-Ethyl-9,10-anthracenedione | | OtherNames = 2-Ethyl-9,10-anthracenedione | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 6514 | | ChemSpiderID = 6514 | ||
| | PubChem = 6772 | |||
| | UNII = 59YJ81QZKD | |||
| | InChI = 1/C16H12O2/c1-2-10-7-8-13-14(9-10)16(18)12-6-4-3-5-11(12)15(13)17/h3-9H,2H2,1H3 | | InChI = 1/C16H12O2/c1-2-10-7-8-13-14(9-10)16(18)12-6-4-3-5-11(12)15(13)17/h3-9H,2H2,1H3 | ||
| | InChIKey = SJEBAWHUJDUKQK-UHFFFAOYAW | | InChIKey = SJEBAWHUJDUKQK-UHFFFAOYAW | ||
| Line 22: | Line 26: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = SJEBAWHUJDUKQK-UHFFFAOYSA-N | | StdInChIKey = SJEBAWHUJDUKQK-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|changed|??}} | ||
| | CASNo = 84-51-5 | | CASNo = 84-51-5 | ||
| | EINECS = 201-535-4 | | EINECS = 201-535-4 | ||
| Line 35: | Line 39: | ||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| ⚫ | | FlashPtC = 155.4 | ||
| | SPhrases = {{S24}} {{S25}} | |||
| | GHSPictograms = {{GHS08}}{{GHS09}} | |||
| ⚫ | | |
||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|350|373|410}} | |||
| | PPhrases = {{P-phrases|201|202|260|273|281|308+313|314|391|405|501}} | |||
| }} | }} | ||
| }} | }} | ||
| '''2-Ethylanthraquinone''' |
'''2-Ethylanthraquinone''' is an ] that is a derivative of ]. This pale yellow solid is used in the industrial production of ] (H<sub>2</sub>O<sub>2</sub>).<ref>{{cite encyclopedia |author1=Goor, G. |author2=Glenneberg, J. |author3=Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 |isbn=9783527303854 }}</ref><ref>Römpp CD 2006, Georg Thieme Verlag 2006</ref> | ||
| ==Production== | ==Production== | ||
| 2-Ethylanthraquinone is prepared from the reaction of ] and ]: C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub> |
2-Ethylanthraquinone is prepared from the reaction of ] and ]: | ||
| :C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub>Et → C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>C<sub>6</sub>H<sub>3</sub>Et + H<sub>2</sub>O. | |||
| Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics. | |||
| ==Uses== | ==Uses== | ||
| ] is produced industrially by the ] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2- |
] is produced industrially by the ] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubstituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or ], process: | ||
| ] |
]{{clear|left}} | ||
| The hydrogenation of ] is catalyzed by ] |
The ] of ] is catalyzed by ]. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hydrogenated but is more difficult to oxidize. The formation of the tetrahydro derivative can be suppressed through the selection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols. | ||
| A working solution must be able to keep the 2-ethylanthraquinone dissolved in the hyrdrogenation, oxidation, and extraction steps. Since hydroquinones dissolve better in polar solvents, and quinones dissolve better in aromatic nonpolar solvents, a solvent mixture must be used. Some suggested mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols. | |||
| ==References== | ==References== | ||
| <references /> | |||
| {{DEFAULTSORT:Ethylanthraquinone, 2-}} | {{DEFAULTSORT:Ethylanthraquinone, 2-}} | ||
Latest revision as of 10:04, 13 January 2024
Intermediate Chemical in H2O2 Synthesis
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Ethylanthracene-9,10-dione | |
| Other names 2-Ethyl-9,10-anthracenedione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.396 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H12O2 |
| Molar mass | 236.27 g/mol |
| Appearance | white to yellowish crystals or powder |
| Density | 1.231g/cm3 |
| Melting point | 105 °C (221 °F; 378 K) |
| Boiling point | 415.4 @ 760mmHg |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H350, H373, H410 |
| Precautionary statements | P201, P202, P260, P273, P281, P308+P313, P314, P391, P405, P501 |
| Flash point | 155.4 °C (311.7 °F; 428.5 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2-Ethylanthraquinone is an organic compound that is a derivative of anthraquinone. This pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).
Production
2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride and ethylbenzene:
- C6H4(CO)2O + C6H5Et → C6H4(CO)2C6H3Et + H2O.
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics.
Uses
Hydrogen peroxide is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubstituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or autoxidation, process:
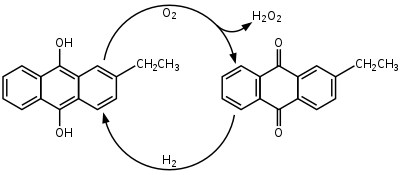
The hydrogenation of 2-ethylanthraquinone is catalyzed by palladium. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hydrogenated but is more difficult to oxidize. The formation of the tetrahydro derivative can be suppressed through the selection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols.
References
- Goor, G.; Glenneberg, J.; Jacobi, S. (2007). "Hydrogen Peroxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_443.pub2. ISBN 9783527303854.
- Römpp CD 2006, Georg Thieme Verlag 2006