| Revision as of 05:13, 24 May 2014 edit99.119.130.240 (talk) nitrogen atmosphere = Inert gas← Previous edit | Latest revision as of 11:55, 6 November 2024 edit undoSpintendo (talk | contribs)Extended confirmed users45,858 edits Numeric names used in author parameters CS1 maintenance message corrected. | ||
| (120 intermediate revisions by 77 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Antibiotic drug introduced in the 1910s}} | |||
| ] ('''A'''), but |
] ('''A'''), but chemical studies published in 2005 suggest<ref name="pmid15624113"/> that salvarsan is actually a mixture of the trimer ('''B''') and the pentamer ('''C''').]] | ||
| '''Arsphenamine''', also known as '''Salvarsan''' |
'''Arsphenamine''', also known as '''Salvarsan''' or '''compound 606''', is an ] ] that was introduced at the beginning of the 1910s as the first effective treatment for the deadly ]s ], ], and ].<ref name='ITOM'>{{Cite book |last1=Gibaud |first1=Stéphane |last2=Jaouen |first2=Gérard |title=Medicinal Organometallic Chemistry |chapter=Arsenic-Based Drugs: From Fowler's Solution to Modern Anticancer Chemotherapy |year=2010 |volume=32 |pages=1–20 |doi= 10.1007/978-3-642-13185-1_1 |series=Topics in Organometallic Chemistry |bibcode=2010moc..book....1G |isbn=978-3-642-13184-4}}</ref><ref>{{Cite book |last1=Ehrlich|first1=Paul|last2=Hata|first2=Sahachiro|last3=Newbold|first3=A.|last4=Felkin|first4=Robert W.|oclc=3225081 |title=The Experimental Chemotherapy of Spirilloses |publisher=Rebman|location=New York |year=1911 |language=en}}</ref> | ||
| This ] was the first modern ].<ref name = Williams /> | |||
| ==History== | ==History== | ||
| {{Main|Magic bullet (medicine)}} | |||
| ] and ] discovered the antisyphilitic activity of this compound in 1909 in Erlich's laboratory, during a survey of hundreds of newly synthesized organic ]al compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered with ] activity without killing the human. Ehrlich's team began their search for such a "]" among chemical derivatives of the dangerously toxic drug ]. This was the first organized team effort to optimize the biological activity of a ] through systematic chemical modifications, the basis for nearly all modern pharmaceutical research. | |||
| ] | |||
| Arsphenamine was first synthesized in 1907 in ]'s lab by ].<ref name = Williams>{{cite journal | pmc = 2726818 | pmid=19679737 | doi=10.1258/jrsm.2009.09k036 | volume=102 | issue=8 | title=The introduction of 'chemotherapy' using arsphenamine - the first magic bullet | year=2009 | journal=J R Soc Med | pages=343–48 | author=Williams KJ}}</ref> The antisyphilitic activity of this compound was discovered by ] in 1909, during a survey of hundreds of newly synthesized organic ]al compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered that would have anti-microbial activity but not kill the human patient. Ehrlich's team began their search for such a "]" among chemical derivatives of the dangerously toxic drug ]. | |||
| Arsphenamine was used to treat the disease ] because it is toxic to the ] '']'', a ] that causes syphilis.<ref>{{Cite journal |last=Abraham |first=J. Johnston |date=December 1948 |title=Some Account of the History of the Treatment of Syphilis* |journal=British Journal of Venereal Diseases |volume=24 |issue=4 |pages=153–160 |doi=10.1136/sti.24.4.153 |issn=0007-134X |pmc=1053609 |pmid=18099878}}</ref> | |||
| ⚫ | Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by ] under the ] |
||
| This instability may due to the fact the true structure of the compound was not confirmed until 2004 | |||
| Ehrlich originally proposed that Salvarsan's structure was of two double-bonded arsenic atoms, each bonded to an aminophenol group. This became the subject of much debate over the years, particularly because of this unlikely double bond between the arsenic atoms. Scientists found that Salvarsan is in fact is a mixture of three- and five-membered cyclic arsenic species.<ref>http://www.rsc.org/chemistryworld/2013/04/salvarsan-podcast</ref> | |||
| ⚫ | Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by ] under the ] "Salvarsan" in 1910.<ref name=acs>{{cite magazine |url=http://pubs.acs.org/cen/coverstory/83/8325/8325salvarsan.html |title=Salvarsan |access-date=2010-02-01 |magazine=] |first=Amanda |last=Yarnell |volume=83 |issue=25 |date=20 June 2005}}</ref><ref>In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is ], which was the 605th compound to be developed in a search for insecticides. It is commonly known as ] (E stands for ''Entwicklungsnummer'', German for "development number").</ref> Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic ] compounds that had been used previously. It was distributed as a yellow, crystalline, ] powder that was highly unstable in air.<ref name=":0">{{cite book |chapter-url=http://chestofbooks.com/health/materia-medica-drugs/American-Medical-Association/A-Handbook-of-Useful-Drugs/Salvarsan-Salvarsan-N-N-R.html |title=A Handbook of Useful Drugs |chapter=Salvarsan, N. N. R. |access-date=17 August 2010 |author=State Medical Examining and Licensing Boards |publisher=Press of the ] |year=1913}}</ref> This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects attributed to Salvarsan, including rashes, liver damage, and risks of life and limb, were thought to be caused by improper handling and administration.<ref>{{cite magazine |url=http://archive.protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars |title=Paul Ehrlich and the Salvarsan Wars |date=Spring 2010 |access-date=21 February 2015 |magazine=] |url-status=dead |archive-url=https://web.archive.org/web/20150221234212/http://archive.protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars |archive-date=21 February 2015}}</ref> This caused Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger."<ref name=acs /> | ||
| ⚫ | Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, ] (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a ] atmosphere to prevent oxidation These arsenical compounds were supplanted as treatments for syphilis in the 1940s by ].<ref> |
||
| ⚫ | Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, ] (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a ] atmosphere to prevent oxidation. These arsenical compounds were supplanted as treatments for syphilis in the 1940s by ].<ref>{{cite journal | pmc = 2790789 | pmid=18679046 | doi=10.1159/000149583 | volume=82 | issue=3 | title=The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize | year=2008 |vauthors=Bosch F, Rosich L | journal=Pharmacology | pages=171–79}}</ref> | ||
| ⚫ | After leaving |
||
| ⚫ | After leaving Ehrlich's laboratory, Hata continued parallel investigation of the new medicines in ].<ref>{{cite journal|author1=Izumi, Yoshio |author2=Isozumi, Kazuo|year=2001|url=http://www.kjm.keio.ac.jp/past/50/2/91.pdf |archive-url=https://web.archive.org/web/20050329181525/http://www.kjm.keio.ac.jp/past/50/2/91.pdf |archive-date=2005-03-29 |url-status=live|format=free download pdf|title=Modern Japanese medical history and the European influence|journal=Keio Journal of Medicine|volume=50|pages=91–99|pmid=11450598|issue=2|doi=10.2302/kjm.50.91|doi-access=free}}</ref> | ||
| ==Mechanism== | |||
| The ] that causes syphilis is a ], '']''. Arsphenamine is not toxic to spirochetes until it has been converted to an active form by the body. | |||
| ==Structure== | ==Structure== | ||
| Salvarsan has long been assumed to have an As=As ], akin to the N=N linkage in ]. However, in 2005, in an extensive ], the arsenic–arsenic bonds in Salvarsan were shown to be single bonds rather than double bonds. Presumed to consist of RAs=AsR molecules, i.e. (RAs)<sub>2</sub>, Salvarsan was found to actually contain a mixture of cyclo-(RAs)<sub>3</sub> and cyclo-(RAs)<sub>5</sub> species, where R is the 3-amino-4-hydroxyphenyl ].<ref name="pmid15624113">{{cite journal |vauthors=Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS |title=The composition of Ehrlich's salvarsan: resolution of a century-old debate |journal=Angew. Chem. Int. Ed. Engl. |volume=44 |issue=6 |pages=941–944 |year=2005 |pmid=15624113 |doi=10.1002/anie.200461471 |hdl=10289/207 |url=https://researchcommons.waikato.ac.nz/bitstream/10289/207/1/content.pdf |archive-url=https://web.archive.org/web/20170506105634/http://researchcommons.waikato.ac.nz/bitstream/10289/207/1/content.pdf |archive-date=2017-05-06 |url-status=live |doi-access=free }}</ref><ref>{{cite web|url = https://www.chemistryworld.com/podcasts/salvarsan/3005937.article|title=Salvarsan (podcast)|date = 22 December 2010|access-date = 16 December 2019|work = ]|publisher = ]|first = Philip|last = Robinson}}</ref> According to Nicholson, these cyclic species slowly release an oxidised species, RAs(OH)<sub>2</sub>, that is likely responsible for Salvarsan's antisyphilis properties.<ref name=acs/> | |||
| ==See also== | ==See also== | ||
| * '']'', 1940 film about Ehrlich's quest to find a cure for syphilis. | * '']'', the 1940 film about Ehrlich's quest to find a cure for syphilis. | ||
| * ], an organoarsenic molecule found in nature with similar activity. | |||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| ] | ] | ||
| Line 30: | Line 31: | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 11:55, 6 November 2024
Antibiotic drug introduced in the 1910s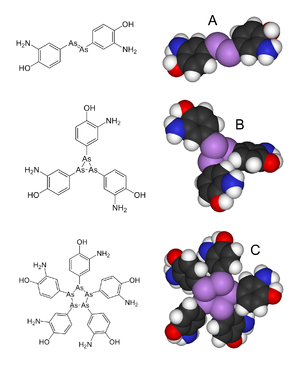
Arsphenamine, also known as Salvarsan or compound 606, is an antibiotic drug that was introduced at the beginning of the 1910s as the first effective treatment for the deadly infectious diseases syphilis, relapsing fever, and African trypanosomiasis. This organoarsenic compound was the first modern antimicrobial agent.
History
Main article: Magic bullet (medicine)
Arsphenamine was first synthesized in 1907 in Paul Ehrlich's lab by Alfred Bertheim. The antisyphilitic activity of this compound was discovered by Sahachiro Hata in 1909, during a survey of hundreds of newly synthesized organic arsenical compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered that would have anti-microbial activity but not kill the human patient. Ehrlich's team began their search for such a "magic bullet" among chemical derivatives of the dangerously toxic drug atoxyl.
Arsphenamine was used to treat the disease syphilis because it is toxic to the bacterium Treponema pallidum, a spirochete that causes syphilis.
Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by Hoechst AG under the trade name "Salvarsan" in 1910. Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic mercury compounds that had been used previously. It was distributed as a yellow, crystalline, hygroscopic powder that was highly unstable in air. This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects attributed to Salvarsan, including rashes, liver damage, and risks of life and limb, were thought to be caused by improper handling and administration. This caused Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger."
Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, Neosalvarsan (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a nitrogen atmosphere to prevent oxidation. These arsenical compounds were supplanted as treatments for syphilis in the 1940s by penicillin.
After leaving Ehrlich's laboratory, Hata continued parallel investigation of the new medicines in Japan.
Structure
Salvarsan has long been assumed to have an As=As double bond, akin to the N=N linkage in azobenzene. However, in 2005, in an extensive mass spectrometric analysis, the arsenic–arsenic bonds in Salvarsan were shown to be single bonds rather than double bonds. Presumed to consist of RAs=AsR molecules, i.e. (RAs)2, Salvarsan was found to actually contain a mixture of cyclo-(RAs)3 and cyclo-(RAs)5 species, where R is the 3-amino-4-hydroxyphenyl moiety. According to Nicholson, these cyclic species slowly release an oxidised species, RAs(OH)2, that is likely responsible for Salvarsan's antisyphilis properties.
See also
- Dr. Ehrlich's Magic Bullet, the 1940 film about Ehrlich's quest to find a cure for syphilis.
- Arsenicin A, an organoarsenic molecule found in nature with similar activity.
References
- ^ Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS (2005). "The composition of Ehrlich's salvarsan: resolution of a century-old debate" (PDF). Angew. Chem. Int. Ed. Engl. 44 (6): 941–944. doi:10.1002/anie.200461471. hdl:10289/207. PMID 15624113. Archived (PDF) from the original on 2017-05-06.
- Gibaud, Stéphane; Jaouen, Gérard (2010). "Arsenic-Based Drugs: From Fowler's Solution to Modern Anticancer Chemotherapy". Medicinal Organometallic Chemistry. Topics in Organometallic Chemistry. Vol. 32. pp. 1–20. Bibcode:2010moc..book....1G. doi:10.1007/978-3-642-13185-1_1. ISBN 978-3-642-13184-4.
- Ehrlich, Paul; Hata, Sahachiro; Newbold, A.; Felkin, Robert W. (1911). The Experimental Chemotherapy of Spirilloses. New York: Rebman. OCLC 3225081.
- ^ Williams KJ (2009). "The introduction of 'chemotherapy' using arsphenamine - the first magic bullet". J R Soc Med. 102 (8): 343–48. doi:10.1258/jrsm.2009.09k036. PMC 2726818. PMID 19679737.
- "Salvarsan treatment kit for syphilis, Germany, 1909–1912 – Wellcome Collection". Wellcome Collection. Retrieved 26 October 2018.
- Abraham, J. Johnston (December 1948). "Some Account of the History of the Treatment of Syphilis*". British Journal of Venereal Diseases. 24 (4): 153–160. doi:10.1136/sti.24.4.153. ISSN 0007-134X. PMC 1053609. PMID 18099878.
- ^ Yarnell, Amanda (20 June 2005). "Salvarsan". Chemical & Engineering News. Vol. 83, no. 25. Retrieved 2010-02-01.
- In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is parathion, which was the 605th compound to be developed in a search for insecticides. It is commonly known as E605 (E stands for Entwicklungsnummer, German for "development number").
- State Medical Examining and Licensing Boards (1913). "Salvarsan, N. N. R.". A Handbook of Useful Drugs. Press of the American Medical Association. Retrieved 17 August 2010.
- "Paul Ehrlich and the Salvarsan Wars". Proto. Spring 2010. Archived from the original on 21 February 2015. Retrieved 21 February 2015.
- Bosch F, Rosich L (2008). "The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize". Pharmacology. 82 (3): 171–79. doi:10.1159/000149583. PMC 2790789. PMID 18679046.
- Izumi, Yoshio; Isozumi, Kazuo (2001). "Modern Japanese medical history and the European influence" (free download pdf). Keio Journal of Medicine. 50 (2): 91–99. doi:10.2302/kjm.50.91. PMID 11450598. Archived (PDF) from the original on 2005-03-29.
- Robinson, Philip (22 December 2010). "Salvarsan (podcast)". Chemistry World. Royal Society of Chemistry. Retrieved 16 December 2019.