| Revision as of 10:10, 12 February 2015 editSeppi333 (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, New page reviewers, Pending changes reviewers, Template editors35,350 edits db← Previous edit | Latest revision as of 20:00, 22 January 2025 edit undoDell Latitude E6400 (talk | contribs)178 editsm Reverted 1 edit by 216.247.20.225 (talk) to last revision by Volun2020Tags: Twinkle Undo | ||
| Line 1: | Line 1: | ||
| {{Short description|Hormone released by the pineal gland}} | |||
| {{Distinguish|Melanotan (disambiguation)|Melanin|Melanopsin|Afamelanotide}} | |||
| {{Hatnote|Not to be confused with ]. For the album, see ''Melatonin'' (album). For the video game see ]}} | |||
| {{drugbox | |||
| {{About|melatonin as a hormone|its role as a supplement and medication|Melatonin as a medication and supplement}} | |||
| | Verifiedfields = changed | |||
| {{cs1 config|name-list-style=vanc}} | |||
| | Watchedfields = changed | |||
| {{Use dmy dates|date=March 2023}} | |||
| | verifiedrevid = 420231802 | |||
| {{Chembox | |||
| | IUPAC_name = ''N''-<br/>acetamide | |||
| | |
<!-- Images -->| ImageFile = Melatonin.svg | ||
| | ImageSize = | |||
| | image2 = Melatonin-3d-CPK.png | |||
| | ImageClass = skin-invert | |||
| | ImageFile2 = Melatonin molecule ball.png | |||
| <!--Clinical data--> | |||
| | ImageSize2 = <!-- Names --> | |||
| | Drugs.com = {{drugs.com|CDI|melatonin}} | |||
| | IUPACName = N-acetamide | |||
| | legal_UK = POM | |||
| | OtherNames = 5-Methoxy-N-acetyltryptamine; N-Acetyl-5-methoxytryptamine; NSC-113928 | |||
| | legal_AU = S4 | |||
| <!-- Sections -->| Section1 = {{Chembox Identifiers | |||
| | legal_US = OTC | |||
| | CASNo = 73-31-4 | |||
| | routes_of_administration = ], ], ] | |||
| | ChEBI = 16796 | |||
| | ChEMBL = 45 | |||
| <!--Pharmacokinetic data--> | |||
| | ChemSpiderID = 872 | |||
| | bioavailability = 30 – 50% | |||
| | DrugBank = DB01065 | |||
| | metabolism = ] via ] mediated 6-hydroxylation | |||
| | EINECS = 200-797-7 | |||
| | elimination_half-life = 35 to 50 minutes | |||
| | |
| EC_number = 200-797-7 | ||
| | InChI = 1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | |||
| | InChIKey = DRLFMBDRBRZALE-UHFFFAOYSA-N | |||
| <!--Identifiers--> | |||
| | KEGG = C01598 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | MeSHName = Melatonin | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 73-31-4 | |||
| | ATC_prefix = N05 | |||
| | ATC_suffix = CH01 | |||
| | PubChem = 896 | | PubChem = 896 | ||
| | SMILES = CC(=O)NCCC1=CNC2=C1C=C(C=C2)OC | |||
| | IUPHAR_ligand = 224 | |||
| | DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| | DrugBank = DB01065 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 872 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = JL5DK93RCL | | UNII = JL5DK93RCL | ||
| }} | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | Section2 = {{Chembox Properties | |||
| | KEGG = D08170 | |||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 16796 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 45 | |||
| <!--Chemical data--> | |||
| | C=13 | H=16 | N=2 | O=2 | | C=13 | H=16 | N=2 | O=2 | ||
| | |
| MolarMass = 232.281 g/mol | ||
| | Appearance = | |||
| | smiles = COC1=CC2=C(NC=C2CCNC(C)=O)C=C1 | |||
| | Density = | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | MeltingPt = 117 | |||
| | StdInChI = 1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | |||
| | BoilingPt = | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | Solubility = | |||
| | StdInChIKey = DRLFMBDRBRZALE-UHFFFAOYSA-N | |||
| }} | |||
| | Section3 = {{Chembox Hazards | |||
| | MainHazards = | |||
| | FlashPt = | |||
| | AutoignitionPt = | |||
| }} | |||
| | Section6 = <!-- {{Chembox Pharmacology | |||
| See and populate infobox at ] rather for pharmacokinetic, pharmacodynamic, etc. | |||
| }} --> | |||
| }} | }} | ||
| '''Melatonin''', an ], is a ] produced by various ], including ] and ].<ref>{{cite journal |doi=10.20945/2359-3997000000066 |title=A brief review about melatonin, a pineal hormone |year=2018 |last1=Amaral |first1=Fernanda Gaspar do |last2=Cipolla-Neto |first2=José |journal=Archives of Endocrinology and Metabolism |volume=62 |issue=4 |pages=472–479 |pmid=30304113 |pmc=10118741 |s2cid=52954755 }}</ref> Its discovery in 1958 by ] and colleagues stemmed from the isolation of a substance from the ] of cows that could induce ] in ]. This compound was later identified as a ] secreted in the ] during the night, playing a crucial role in regulating the ], also known as the circadian rhythm, in ].<ref name="Auld2017">{{cite journal | vauthors = Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL | title = Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders | journal = Sleep Medicine Reviews | volume = 34 | pages = 10–22 | date = August 2017 | pmid = 28648359 | doi = 10.1016/j.smrv.2016.06.005 | hdl = 20.500.11820/0e890bda-4b1d-4786-a907-a03b1580fd07 | url = http://epubs.surrey.ac.uk/813219/1/Riha%20accepted%20MS%202016.pdf | hdl-access = free }}</ref><ref>{{cite book | vauthors = Faraone SV |title=ADHD: Non-Pharmacologic Interventions, An Issue of Child and Adolescent Psychiatric Clinics of North America, E-Book |date=2014 |publisher=Elsevier Health Sciences |isbn=978-0-323-32602-5 |page=888 |url=https://books.google.com/books?id=lNSlBAAAQBAJ&pg=PA888}}</ref> | |||
| '''Melatonin''' ({{IPAc-en|audio=melatonin-pronunciation.ogg|ˌ|m|ɛ|l|ə|ˈ|t|oʊ|n|ɪ|n}}), chemically '''''N''-]-5-]]''',<ref>{{cite web| title=Melatonin| publisher=Sleepdex| accessdate=2011-08-17| url=http://www.sleepdex.org/melatonin.htm}}</ref> | |||
| is a ] found in animals, plants, fungi and bacteria.<ref>{{cite journal|last1=Hardeland|first1=Rüdiger|last2=Pandi-Perumal|first2=S.R.|last3=Cardinali|first3=Daniel P.|title=Melatonin|journal=The International Journal of Biochemistry & Cell Biology|date=2006|volume=38|issue=3|pages=313–316|doi=10.1016/j.biocel.2005.08.020|pmid=16219483}}</ref> | |||
| It is synthesized in animal cells directly from the ] ], but in other organisms through the ], in response to dark-light periods (]).<ref>{{cite journal|last1=Tan|first1=Dun-Xian|last2=Zheng|first2=Xiaodong|last3=Kong| first3=Jin|last4=Manchester|first4=Lucien |last5=Hardeland| first5=Ruediger|last6=Kim|first6=Seok|last7=Xu|first7=Xiaoying|last8=Reiter|first8=Russel|title=Fundamental Issues Related to the Origin of Melatonin and Melatonin Isomers during Evolution: Relation to Their Biological Functions|journal=International Journal of Molecular Sciences|date=9 September 2014|volume=15|issue=9|pages=15858–15890|doi=10.3390/ijms150915858|pmid=25207599|pmc=4200856}}</ref> | |||
| <ref name=acuna>{{cite book|last1=Acuna-Castroviejo|first1=D|last2=Escames|first2=G|last3=Tapias|first3=V|last4=Rivas|first4=I|editor1-last=Montilla|editor1-first=Pedro|editor2-last=Túnez|editor2-first=Isaac|title=Melatonin: Present and Future|date=2006|publisher=Nova Science Publishers|location=New York, US|isbn=9781600213748|pages=1-33|url=http://books.google.co.in/books?id=cQn9NNUinwYC&printsec|chapter=Melatonin, mitochondria and neuroprotection}}</ref> | |||
| In vertebrates, melatonin's functions extend to ] sleep-wake cycles, encompassing sleep-wake timing and ], as well as controlling seasonal rhythmicity (]), which includes reproduction, fattening, molting, and hibernation.<ref name="Altun2007">{{cite journal | vauthors = Altun A, Ugur-Altun B | title = Melatonin: therapeutic and clinical utilization | journal = International Journal of Clinical Practice | volume = 61 | issue = 5 | pages = 835–45 | date = May 2007 | pmid = 17298593 | doi = 10.1111/j.1742-1241.2006.01191.x | s2cid = 18050554 | doi-access = free }}</ref> Its effects are mediated through the activation of ]s and its role as an ].<ref name="Boutin2005">{{cite journal |vauthors=Boutin JA, Audinot V, Ferry G, Delagrange P |date=August 2005 |title=Molecular tools to study melatonin pathways and actions |journal=Trends in Pharmacological Sciences |volume=26 |issue=8 |pages=412–9 |doi=10.1016/j.tips.2005.06.006 |pmid=15992934}}</ref><ref name="Hardeland2005">{{cite journal |vauthors=Hardeland R |date=July 2005 |title=Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance |journal=Endocrine |volume=27 |issue=2 |pages=119–30 |doi=10.1385/ENDO:27:2:119 |pmid=16217125 |s2cid=46984486}}</ref><ref name="Reiter2001">{{cite journal |vauthors=Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S |date=June 2001 |title=Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system |journal=Annals of the New York Academy of Sciences |volume=939 |issue=1 |pages=200–15 |bibcode=2001NYASA.939..200R |doi=10.1111/j.1749-6632.2001.tb03627.x |pmid=11462772 |s2cid=20404509}}</ref> In plants and bacteria, melatonin primarily serves as a defense mechanism against ], indicating its evolutionary significance.<ref name="Tan_2012">{{cite journal |vauthors=Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ |date=January 2012 |title=Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science |journal=Journal of Experimental Botany |volume=63 |issue=2 |pages=577–97 |doi=10.1093/jxb/err256 |pmid=22016420 |doi-access=free}}</ref> The ], key ] within cells, are the main producers of antioxidant melatonin,<ref>{{Cite journal |last1=Reiter |first1=Russel J. |last2=Tan |first2=Dun Xian |last3=Rosales-Corral |first3=Sergio |last4=Galano |first4=Annia |last5=Zhou |first5=Xin Jia |last6=Xu |first6=Bing |date=2018 |title=Mitochondria: Central Organelles for Melatonin's Antioxidant and Anti-Aging Actions |journal=Molecules |volume=23 |issue=2 |pages=509 |doi=10.3390/molecules23020509 |pmc=6017324 |pmid=29495303 |doi-access=free }}</ref> underscoring the molecule's "ancient origins" and its fundamental role in protecting the earliest cells from ].<ref name=":0">{{Cite journal |last1=Manchester |first1=Lucien C. |last2=Coto-Montes |first2=Ana |last3=Boga |first3=Jose Antonio |last4=Andersen |first4=Lars Peter H. |last5=Zhou |first5=Zhou |last6=Galano |first6=Annia |last7=Vriend |first7=Jerry |last8=Tan |first8=Dun-Xian |last9=Reiter |first9=Russel J. |date=2015 |title=Melatonin: an ancient molecule that makes oxygen metabolically tolerable |journal=Journal of Pineal Research |volume=59 |issue=4 |pages=403–419 |doi=10.1111/jpi.12267 |pmid=26272235|s2cid=24373303 |doi-access=free }}</ref><ref name=":1">{{Cite journal |last1=Zhao |first1=Dake |last2=Yu |first2=Yang |last3=Shen |first3=Yong |last4=Liu |first4=Qin |last5=Zhao |first5=Zhiwei |last6=Sharma |first6=Ramaswamy |last7=Reiter |first7=Russel J. |date=2019 |title=Melatonin Synthesis and Function: Evolutionary History in Animals and Plants |journal=Frontiers in Endocrinology |volume=10 |pages=249 |doi=10.3389/fendo.2019.00249 |pmc=6481276 |pmid=31057485 |doi-access=free }}</ref> | |||
| In animals, melatonin controls the daily night-day cycle, thereby allowing the ] of the ]s of several biological functions.<ref name="Altun2007">{{cite journal | author = Altun A, Ugur-Altun B | title = Melatonin: therapeutic and clinical utilization | journal = Int. J. Clin. Pract. | volume = 61 | issue = 5 | pages = 835–45 | date = May 2007 | pmid = 17298593 | doi = 10.1111/j.1742-1241.2006.01191.x }}</ref> | |||
| Many biological effects of melatonin are produced through activation of ]s,<ref name="Boutin2005">{{cite journal | author = Boutin JA, Audinot V, Ferry G, Delagrange P | title = Molecular tools to study melatonin pathways and actions | journal = Trends Pharmacol. Sci. | volume = 26 | issue = 8 | pages = 412–9 | date = August 2005 | pmid = 15992934 | doi = 10.1016/j.tips.2005.06.006 }}</ref> | |||
| while others are due to its role as a pervasive and powerful ],<ref name="Hardeland2005">{{cite journal | author = Hardeland R | title = Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance | journal = Endocrine | volume = 27 | issue = 2 | pages = 119–30 | date = July 2005 | pmid = 16217125 | doi = 10.1385/ENDO:27:2:119 }}</ref> | |||
| with a particular role in the protection of ] and ].<ref name="Reiter2001">{{cite journal | author = Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S | title = Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system | journal = Ann. N. Y. Acad. Sci. | volume = 939 | issue = | pages = 200–15 | date = June 2001 | pmid = 11462772 | doi = 10.1111/j.1749-6632.2001.tb03627.x }}</ref> | |||
| In addition to its ] functions as a hormone and antioxidant, melatonin is also administered exogenously as a ]. It is utilized in the treatment of ]s, including ] and various ].{{TOC limit}} | |||
| The hormone can be used as a sleep aid and in the treatment of ]s. It can be taken orally as capsules, tablets, or liquid. It is also available in a form to be used sublingually, and there are transdermal patches. There have been few long-term clinical trials in the use of melatonin in humans. | |||
| ==Biological activity== | |||
| ==Discovery== | |||
| In humans, melatonin primarily acts as a potent ] of two types of ]: ], with ] ], and ], with nanomolar binding affinity. Both receptors are part of the ] (GPCRs) family, specifically the ] GPCRs,<ref name="IUPHAR – melatonin receptors review">{{cite journal | vauthors = Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, Cecon E, Zlotos DP | display-authors = 6 | title = Update on melatonin receptors: IUPHAR Review 20 | journal = British Journal of Pharmacology | volume = 173 | issue = 18 | pages = 2702–25 | date = September 2016 | pmid = 27314810 | pmc = 4995287 | doi = 10.1111/bph.13536 | quote = Hence, one melatonin molecule and its associated metabolites could scavenge a large number of reactive species, and thus, the overall antioxidant capacity of melatonin is believed to be greater than that of other well-known antioxidants, such as vitamin C and vitamin E, under in vitro or in vivo conditions (Gitto et al., 2001; Sharma and Haldar, 2006; Ortiz et al., 2013). }}</ref><ref>{{cite web|url=http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39|title=Melatonin receptors {{!}} G protein-coupled receptors {{!}} IUPHAR/BPS Guide to Pharmacology|website=www.guidetopharmacology.org|access-date=7 April 2017}}</ref> although melatonin receptor 1 also exhibits coupling with ].<ref name="IUPHAR – melatonin receptors review" /> | |||
| Furthermore, melatonin functions as a high-capacity ], or free radical scavenger, within ], playing a dual role in combating cellular ]. First, it directly neutralizes ], and second, it promotes the ] of essential antioxidant enzymes, such as ], ], ], and ]. This increase in antioxidant enzyme expression is mediated through ] activated by the binding of melatonin to its receptors. Through these mechanisms, melatonin protects the cell against oxidative stress in two ways, and plays other roles in human health than only regulating the sleep-wake cycle.<ref name="pmid28400824">{{cite journal |vauthors=Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A |date=2017 |title=Melatonin and human mitochondrial diseases |journal=Journal of Research in Medical Sciences |volume=22 |pages=2 |doi=10.4103/1735-1995.199092 |pmc=5361446 |pmid=28400824 |doi-access=free}}</ref><ref name="IUPHAR – melatonin receptors review" /><ref name="Melatonin as a mitochondrial antioxidant – 2017 Review">{{cite journal |vauthors=Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B |date=November 2017 |title=Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas |journal=Cellular and Molecular Life Sciences |volume=74 |issue=21 |pages=3863–3881 |doi=10.1007/s00018-017-2609-7 |pmid=28864909 |s2cid=23820389 |quote=melatonin is specifically targeted to the mitochondria where it seems to function as an apex antioxidant ... The measurement of the subcellular distribution of melatonin has shown that the concentration of this indole in the mitochondria greatly exceeds that in the blood.|pmc=11107735 }}</ref><ref name="Melatonin – 2016 Review">{{cite journal |vauthors=Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L |date=October 2016 |title=Melatonin as an antioxidant: under promises but over delivers |journal=Journal of Pineal Research |volume=61 |issue=3 |pages=253–78 |doi=10.1111/jpi.12360 |pmid=27500468 |s2cid=35435683 |quote=There is credible evidence to suggest that melatonin should be classified as a mitochondria-targeted antioxidant. |doi-access=free}}</ref><ref name="pmid26272235">{{cite journal |display-authors=6 |vauthors=Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ |date=November 2015 |title=Melatonin: an ancient molecule that makes oxygen metabolically tolerable |journal=Journal of Pineal Research |volume=59 |issue=4 |pages=403–19 |doi=10.1111/jpi.12267 |pmid=26272235 |s2cid=24373303 |quote=While originally thought to be produced exclusively in and secreted from the vertebrate pineal gland , it is now known that the indole is present in many, perhaps all, vertebrate organs and in organs of all plants that have been investigated . That melatonin is not relegated solely to the pineal gland is also emphasized by the reports that it is present in invertebrates , which lack a pineal gland and some of which consist of only a single cell. |doi-access=free}}</ref><ref name="Melatonin transporters – 2017 review">{{cite journal |vauthors=Mayo JC, Sainz RM, González-Menéndez P, Hevia D, Cernuda-Cernuda R |date=November 2017 |title=Melatonin transport into mitochondria |journal=Cellular and Molecular Life Sciences |volume=74 |issue=21 |pages=3927–3940 |doi=10.1007/s00018-017-2616-8 |pmid=28828619 |s2cid=10920415|pmc=11107582 }}</ref> | |||
| Melatonin was first discovered in connection to the mechanism by which some ] and ] change the color of their skin.<ref name="Filadelfi1996">{{cite journal | author = Filadelfi AM, Castrucci AM | title = Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates | journal = J. Pineal Res. | volume = 20 | issue = 4 | pages = 175–86 | date = May 1996 | pmid = 8836950 | doi = 10.1111/j.1600-079X.1996.tb00256.x }}</ref> | |||
| <ref name="Sugden2004">{{cite journal | author = Sugden D, Davidson K, Hough KA, Teh MT | title = Melatonin, melatonin receptors and melanophores: a moving story | journal = Pigment Cell Res. | volume = 17 | issue = 5 | pages = 454–60 | date = October 2004 | pmid = 15357831 | doi = 10.1111/j.1600-0749.2004.00185.x }}</ref> | |||
| As early as 1917, Carey Pratt McCord and Floyd P. Allen discovered that feeding extract of the ]s of cows lightened tadpole skin by contracting the dark ] ].<ref name="Coates">{{cite book | author = Coates PM, Blackman MR, Cragg GM, LevineM, Moss J, White JD| title = Encyclopedia of dietary supplements | edition = | language = | publisher = Marcel Dekker | location = New York, N.Y | year = 2005 | origyear = | pages = 457–66 | quote = | isbn = 0-8247-5504-9 | url = http://books.google.com/?id=Sfmc-fRCj10C&pg=PA457&lpg=PA457&dq=Lerner+melatonin+history }}</ref> | |||
| <ref name="McCord">{{cite journal |author=McCord CP, Allen FP |title=Evidences associating pineal gland function with alterations in pigmentation |date=January 1917 |journal=J Exptl Zool |volume= 23 |issue=1 |pages=206–24 |url=http://books.google.com/?id=OOM1AQAAMAAJ&pg=PA207 |doi=10.1002/jez.1400230108 }}</ref> | |||
| ==Biological functions== | |||
| In 1958 dermatology professor ] and colleagues at Yale University, in the hope that a substance from the pineal might be useful in treating skin diseases, isolated the hormone from bovine ] extracts and named it ''melatonin''.<ref name="pmid14415935">{{cite journal | author = Lerner AB, Case JD, Takahashi Y | title = Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands | journal = J. Biol. Chem. | volume = 235 | issue = | pages = 1992–7 | date = July 1960 | pmid = 14415935 | doi = }}</ref> | |||
| ] | |||
| In the mid-70s Lynch ''et al.'' demonstrated<ref name="pmid1167425">{{cite journal | author = Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH | title = Daily rhythm in human urinary melatonin | journal = Science | volume = 187 | issue = 4172 | pages = 169–71 | date = January 1975 | pmid = 1167425 | doi = 10.1126/science.1167425 | bibcode = 1975Sci...187..169L }}</ref> | |||
| that the production of melatonin exhibits a ] in human pineal glands. | |||
| ===Circadian rhythm=== | |||
| The discovery that melatonin is an antioxidant was made in 1993.<ref name="scavenger">{{cite journal | author = Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC | title = Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis | journal = J. Pineal Res. | volume = 14 | issue = 4 | pages = 151–68 | date = May 1993 | pmid = 8102180 | doi = 10.1111/j.1600-079X.1993.tb00498.x | url = }}</ref> | |||
| {{Main|Circadian rhythm}} | |||
| The first ] for its use as a low dose sleep aid was granted to ] at ] in 1995.<ref>{{ cite patent | country = US | number = 5449683 | status = patent | title = Methods of inducing sleep using melatonin | gdate = 1995-09-12 | fdate = 1993-07-16 | inventor = Wurtman RJ | assign1 = Massachusetts Institute of Technology }}</ref> Around the same time, the hormone got a lot of press as a possible treatment for many illnesses.<ref name="pmid16077149">{{cite journal | author = Arendt J | title = Melatonin: characteristics, concerns, and prospects | journal = J. Biol. Rhythms | volume = 20 | issue = 4 | pages = 291–303 | date = August 2005 | pmid = 16077149 | doi = 10.1177/0748730405277492 | quote = There is very little evidence in the short term for toxicity or undesirable effects in humans. The extraordinary “hype” of the miraculous powers of melatonin in the recent past did a disservice to acceptance of its genuine benefits. }}</ref> | |||
| In mammals, melatonin is critical for the regulation of sleep–wake cycles, or circadian rhythms.<ref name="EmetM">{{cite journal | vauthors = Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A | title = A Review of Melatonin, Its Receptors and Drugs | journal = The Eurasian Journal of Medicine | volume = 48 | issue = 2 | pages = 135–41 | date = June 2016 | pmid = 27551178 | pmc = 4970552 | doi = 10.5152/eurasianjmed.2015.0267 }}</ref> The establishment of regular melatonin levels in human infants occurs around the third month after birth, with ] observed between midnight and 8:00 am.<ref name="pmid12589109">{{cite journal | vauthors = Ardura J, Gutierrez R, Andres J, Agapito T | title = Emergence and evolution of the circadian rhythm of melatonin in children | journal = Hormone Research | volume = 59 | issue = 2 | pages = 66–72 | year = 2003 | pmid = 12589109 | doi = 10.1159/000068571 | doi-broken-date = 10 December 2024 | s2cid = 41937922 }}</ref> It has been documented that melatonin production diminishes as a person ages.<ref name="pmid3783419">{{cite journal | vauthors = Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM | title = Human melatonin production decreases with age | journal = Journal of Pineal Research | volume = 3 | issue = 4 | pages = 379–88 | year = 1986 | pmid = 3783419 | doi = 10.1111/j.1600-079X.1986.tb00760.x | s2cid = 33664568 }}</ref> Additionally, a shift in the timing of melatonin secretion is observed during adolescence, resulting in delayed sleep and wake times, increasing their risk for ] during this period.<ref>{{cite journal | vauthors = Hagenauer MH, Perryman JI, Lee TM, Carskadon MA | title = Adolescent changes in the homeostatic and circadian regulation of sleep | journal = Developmental Neuroscience | volume = 31 | issue = 4 | pages = 276–84 | date = June 2009 | pmid = 19546564 | pmc = 2820578 | doi = 10.1159/000216538 }}</ref> | |||
| ''The New England Journal of Medicine'' editorialized in 2000: "The hype and the claims of the so-called miraculous powers of melatonin several years ago did a great disservice to a scientific field of real importance to human health. With these recent careful and precise observations in blind persons, the true potential of melatonin is becoming evident, and the importance of the timing of treatment is becoming clear. Our 24-hour society, with its chaotic time cues and lack of natural light, may yet reap substantial benefits."<ref name="pmid11027748">{{cite journal | author = Arendt J | title = Melatonin, circadian rhythms, and sleep | journal = N. Engl. J. Med. | volume = 343 | issue = 15 | pages = 1114–6 | date = October 2000 | pmid = 11027748 | doi = 10.1056/NEJM200010123431510 }}</ref> | |||
| The antioxidant properties of melatonin were first recognized in 1993.<ref>{{cite journal | vauthors = Tan DX, Chen LD, Poeggeler B, L Manchester C, Reiter RJ | title = Melatonin: a potent, endogenous hydroxyl radical scavenger. | journal = Endocr. J. | date = 1993 | volume = 1 | pages = 57–60 | url = https://docs.google.com/document/d/e/2PACX-1vSHolKyTREzsC-RB0H-brwbUhaVP4EZBRSoZ6F7b4cOcAkutpNX3ebh0yd_QKEWRBTYVLcqpmMit3NL/pub }}</ref> ] studies reveal that melatonin directly neutralizes various ], including ] (OH•), ] (O2−•), and ] such as ] (NO•).<ref name="pmid7832450">{{cite journal |vauthors=Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR |date=November 1994 |title=Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro |journal=Annals of the New York Academy of Sciences |volume=738 |issue=1 |pages=419–20 |bibcode=1994NYASA.738..419P |doi=10.1111/j.1749-6632.1994.tb21831.x |pmid=7832450 |s2cid=36383425}}</ref><ref name="Melatonin Plants">{{cite journal |vauthors=Arnao MB, Hernández-Ruiz J |date=May 2006 |title=The physiological function of melatonin in plants |journal=Plant Signaling & Behavior |volume=1 |issue=3 |pages=89–95 |bibcode=2006PlSiB...1...89A |doi=10.4161/psb.1.3.2640 |pmc=2635004 |pmid=19521488}}</ref> In plants, melatonin works ] with other antioxidants, enhancing the overall effectiveness of each antioxidant.<ref name="Melatonin Plants" /> This compound has been found to be twice as efficacious as ], a known potent ] antioxidant, at scavenging peroxyl radicals.<ref name="pmid7934611">{{cite journal | vauthors = Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F | title = Melatonin: a peroxyl radical scavenger more effective than vitamin E | journal = Life Sciences | volume = 55 | issue = 15 | pages = PL271-6 | year = 1994 | pmid = 7934611 | doi = 10.1016/0024-3205(94)00666-0 }}</ref> The promotion of antioxidant enzyme expression, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase, is mediated through melatonin receptor-triggered signal transduction pathways.<ref name="IUPHAR – melatonin receptors review" /><ref name="pmid28400824" /> | |||
| ==Biosynthesis and Pharmacology== | |||
| Melatonin biosynthesis in humans and some other organisms involves four enzymatic steps from the essential dietary amino acid ], which follows a ]. | |||
| Melatonin's concentration in the ] is significantly higher than that found in the ],<ref name="Melatonin as a mitochondrial antioxidant – 2017 Review" /><ref name="Melatonin – 2016 Review" /><ref name="pmid26272235" /> emphasizing its role not only in direct free radical scavenging but also in modulating the expression of antioxidant enzymes and maintaining mitochondrial integrity. This multifaceted role shows the physiological significance of melatonin as a mitochondrial antioxidant, a notion supported by numerous scholars.<ref name="pmid28400824" /><ref name="Melatonin as a mitochondrial antioxidant – 2017 Review" /><ref name="Melatonin – 2016 Review" /><ref name="pmid26272235" /><ref name="Melatonin transporters – 2017 review" /> | |||
| In the first two steps, L-tryptophan is first converted to 5-hydroxy-L-tryptophan (5-HTP) by an enzyme, tryptophan 5-hydroxylase. | |||
| 5-HTP is then decarboxylated (] removal) by 5-hydroxytryptophan decarboxylase to produce ]. This point is the rate limiting stage such that further reaction is determined by light-dark conditions. | |||
| Furthermore, the interaction of melatonin with reactive oxygen and nitrogen species results in the formation of metabolites capable of reducing free radicals.<ref name="IUPHAR – melatonin receptors review" /><ref name="Melatonin transporters – 2017 review" /> These metabolites, including ], ] (AFMK), and ] (AMK), contribute to the broader antioxidative effects of melatonin through further ] with free radicals.<ref name="IUPHAR – melatonin receptors review" /><ref name="Melatonin transporters – 2017 review" /> | |||
| Only in darkness, the key enzyme, ] (AANAT) is activated and converts serotonin to N-acetyl serotonin, which is ultimately converted to melatonin by the final enzyme, acetylserotonin O-methyltransferase.<ref>{{cite web|title=MetaCyc Pathway: serotonin and melatonin biosynthesis|url=http://www.metacyc.org/META/new-image?object=PWY-6030|website=MetaCyc.org|publisher=SRI International|accessdate=2 November 2014}}</ref><ref name=norman>{{cite book|last1=Norman|first1=Anthony W.|last2=Henry|first2=Helen L.|title=Hormones|date=2012|publisher=Academic Press|location=Oxford, UK|isbn=978-0-12-369444-7|pages=352-359|edition=3|url=http://books.google.co.in/books?id=_renonjXq68C&dq}}</ref> | |||
| It is the key regulator of melatonin synthesis from tryptophan, as its gene '']'' is directly influenced by photoperiod. | |||
| In humans, 90% of melatonin is cleared in a single passage through the liver, a small amount is excreted in urine,<ref name=USAHRQ /> and a small amount is found in saliva. | |||
| In bacteria, protists, fungi, and plants melatonin is synthesized indirectly with tryptophan as an intermediate product of the ]. In these cells synthesis starts with d-erythrose-4-phosphate and ], and in ] with carbon dioxide. The rest of the reactions are similar, but with slight variations in the last two enzymes.<ref>{{cite journal|last1=Bochkov|first1=Denis V.|last2=Sysolyatin|first2=Sergey V.|last3=Kalashnikov|first3=Alexander I.|last4=Surmacheva|first4=Irina A.|title=Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources|journal=Journal of Chemical Biology|date=2011|volume=5|issue=1|pages=5–17|doi=10.1007/s12154-011-0064-8|pmid=22826715|pmc=3251648}}</ref><ref>{{cite journal|last1=Hardeland|first1=R.|title=Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions|journal=Journal of Experimental Botany|date=2014|volume=18|issue=pii|pages=eru386|doi=10.1093/jxb/eru386|pmid=25240067}}</ref> | |||
| ===Regulation=== | |||
| In vertebrates, melatonin secretion is regulated by ]. Norepinephrine elevates the intracellular cAMP concentration via beta-adrenergic receptors and activates the cAMP-dependent protein kinase A (PKA). PKA phosphoryates the penultimate enzyme, the arylalkylamine N-acetyltransferase (AANAT). At daylight, noradrenergic stimulation stops and the protein is immediately destroyed by ] ].<ref>{{cite journal|last1=Schomerus|first1=C.|last2=Korf|first2=HW|title=Mechanisms regulating melatonin synthesis in the mammalian pineal organ|journal=Annals of the New York Academy of Sciences|date=2005|volume=1057|issue=1|pages=372–383|doi=10.1196/annals.1356.028|pmid=16399907}}</ref> Production is again started in the evening, which is called the dim-light melatonin onset (DLMO). | |||
| It is principally blue light, around 460 to 480 ], that suppresses melatonin,<ref name="Brainard 2001">{{cite journal | author = Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD | title = Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor | journal = J. Neurosci. | volume = 21 | issue = 16 | pages = 6405–12 | date = August 2001 | pmid = 11487664 | doi = }}</ref> | |||
| proportional to the light intensity and length of exposure. Until recent history, humans in temperate climates were exposed to few hours of (blue) daylight in the winter; their fires gave predominantly yellow light. The ] widely used in the twentieth century produced relatively little blue light.<ref></ref> | |||
| Wearing glasses that block blue light in the hours before bedtime may decrease melatonin loss. Kayumov ''et al.'' showed that light containing only wavelengths greater than 530 nm does not suppress melatonin in bright-light conditions.<ref name="Kayumov 2005">{{cite journal | author = Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM | title = Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work | journal = J. Clin. Endocrinol. Metab. | volume = 90 | issue = 5 | pages = 2755–61 | date = May 2005 | pmid = 15713707 | doi = 10.1210/jc.2004-2062 }}</ref> | |||
| Use of blue-blocking goggles the last hours before bedtime has also been advised for people who need to adjust to an earlier bedtime, as melatonin promotes sleepiness.<ref>{{cite journal | author = Burkhart K, Phelps JR | title = Amber lenses to block blue light and improve sleep: a randomized trial | journal = Chronobiol Int | volume = 26 | issue = 8 | pages = 1602–12 | date = 26 December 2009 | pmid = 20030543 | doi = 10.3109/07420520903523719 }}</ref> | |||
| When used several hours before sleep according to the ] for melatonin in humans, small amounts (0.3 mg<ref name="pmid16295212"/>) of melatonin shift the circadian clock earlier, thus promoting earlier sleep onset and morning awakening.<ref name="isbn3-8055-9120-9">{{cite book | author = Terman MR, Wirz-Justice A | title = Chronotherapeutics for Affective Disorders: A Clinician's Manual for Light and Wake Therapy | publisher = S Karger Pub | location = Basel | year = 2009 | page = 71 | isbn = 3-8055-9120-9 }}</ref> | |||
| == Animals == | |||
| In vertebrates, melatonin is produced at nighttime by the pineal gland, a small endocrine gland<ref name="Reiter">{{cite journal | author = Reiter RJ | title = Pineal melatonin: cell biology of its synthesis and of its physiological interactions | journal = Endocr. Rev. | volume = 12 | issue = 2 | pages = 151–80 | date = May 1991 | pmid = 1649044 | doi = 10.1210/edrv-12-2-151 }}</ref> | |||
| located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the ] (SCN) from retinal ]s of the eyes.<ref name="Richardson2005">{{cite journal | author = Richardson GS | title = The human circadian system in normal and disordered sleep | journal = J Clin Psychiatry | volume = 66 Suppl 9 | issue = | pages = 3–9; quiz 42–3 | year = 2005 | pmid = 16336035 | doi = }}</ref><ref name="Perreau-Lenz2004">{{cite journal | author = Perreau-Lenz S, Pévet P, Buijs RM, Kalsbeek A | title = The biological clock: the bodyguard of temporal homeostasis | journal = Chronobiol. Int. | volume = 21 | issue = 1 | pages = 1–25 | date = January 2004 | pmid = 15129821 | doi = 10.1081/CBI-120027984 }}</ref> | |||
| rather than the melatonin signal (as was once postulated). Many animals use the variation in duration of melatonin production each day as a seasonal clock.<ref name="Lincoln2003">{{cite journal | author = Lincoln GA, Andersson H, Loudon A | title = Clock genes in calendar cells as the basis of annual timekeeping in mammals – a unifying hypothesis | journal = J. Endocrinol. | volume = 179 | issue = 1 | pages = 1–13 | date = October 2003 | pmid = 14529560 | doi = 10.1677/joe.0.1790001 }}</ref> In animals including humans<ref name="Arendt2005">{{cite journal | author = Arendt J, Skene DJ | title = Melatonin as a chronobiotic | journal = Sleep Med Rev | volume = 9 | issue = 1 | pages = 25–39 | date = February 2005 | pmid = 15649736 | doi = 10.1016/j.smrv.2004.05.002 | quote = Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn) it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times. }}</ref> | |||
| the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (]) seasonal functions such as reproduction, behavior, coat growth and camouflage ] in seasonal animals.<ref name="Arendt2005"/> In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including mynah birds<ref name="Chaturvedi">{{cite journal | pages = 803–9 | doi = 10.1071/ZO9840803 | url = http://www.publish.csiro.au/paper/ZO9840803.htm | title = Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis | year = 1984 | author = Chaturvedi CM | journal = Australian Journal of Zoology | volume = 32 | issue = 6}}</ref> | |||
| and hamsters.<ref name="Chen1981">{{cite journal | author = Chen HJ | title = Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition | journal = Neuroendocrinology | volume = 33 | issue = 1 | pages = 43–6 | date = July 1981 | pmid = 7254478 | doi = 10.1159/000123198 }}</ref> | |||
| Melatonin can suppress ] by inhibiting secretion of ] (LH) and ] (FSH) from the ] gland, especially in mammals that have a ] season when daylight hours are long. The reproduction of ] is ] and the reproduction of ] is stimulated by melatonin. During the night, melatonin regulates ], lowering its levels. | |||
| == Plants == | |||
| Melatonin is identified in many plants including ] (''Tanacetum parthenium''), ] (''Hypericum perforatum''),<ref name="Paredes2009">{{cite journal | author = Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ | title = Phytomelatonin: a review | journal = J. Exp. Bot. | volume = 60 | issue = 1 | pages = 57–69 | year = 2009 | pmid = 19033551 | doi = 10.1093/jxb/ern284 }}</ref> | |||
| rice, corn, tomato, grape<ref name="pmid19445314">{{cite journal | author = Iriti M, Faoro F | title = Bioactivity of grape chemicals for human health | journal = Nat Prod Commun | volume = 4 | issue = 5 | pages = 611–34 | year = 2009 | pmid = 19445314 | doi = }}</ref> | |||
| and other edible fruits.<ref name="pmid22016420">{{cite journal | author = Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ | title = Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science | journal = J. Exp. Bot. | volume = 63 | issue = 2 | pages = 577–97 | date = January 2012 | pmid = 22016420 | doi = 10.1093/jxb/err256 }}</ref> | |||
| The physiological roles in plants include regulation of their response to ], defense against harsh environments, and the function of an antioxidant.<ref name="pmid20039865">{{cite journal | author = Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ | title = The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness | journal = Biol Rev Camb Philos Soc | volume = 85 | issue = 3 | pages = 607–23 | date = August 2010 | pmid = 20039865 | doi = 10.1111/j.1469-185X.2009.00118.x }}</ref> | |||
| It also regulates plant growth by its ability to slow root formation, while promoting above-ground growth.<ref name="Melatonin Plants">{{cite journal | author = Arnao MB, Hernández-Ruiz J | title = The physiological function of melatonin in plants | journal = Plant Signal Behav | volume = 1 | issue = 3 | pages = 89–95 | date = May 2006 | pmid = 19521488 | pmc = 2635004 | doi = 10.4161/psb.1.3.2640 }}</ref> | |||
| ==Functions== | |||
| ===Circadian rhythm=== | |||
| In animals, the primary function is regulation of day-night cycles. Human infants' melatonin levels become regular in about the third month after birth, with the highest levels measured between midnight and 8:00 AM.<ref name="pmid12589109">{{cite journal | author = Ardura J, Gutierrez R, Andres J, Agapito T | title = Emergence and evolution of the circadian rhythm of melatonin in children | journal = Horm. Res. | volume = 59 | issue = 2 | pages = 66–72 | year = 2003 | pmid = 12589109 | doi = 10.1159/000068571 }}</ref> Human melatonin production decreases as a person ages.<ref name="pmid3783419">{{cite journal | author = Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM | title = Human melatonin production decreases with age | journal = J. Pineal Res. | volume = 3 | issue = 4 | pages = 379–88 | year = 1986 | pmid = 3783419 | doi = 10.1111/j.1600-079X.1986.tb00760.x }}</ref> Also, as children become teenagers, the nightly schedule of melatonin release is delayed, leading to later sleeping and waking times.<ref>{{cite web | title = Why Aren't Teens Getting Enough Sleep? | url = http://kidshealth.org/teen/your_body/take_care/how_much_sleep.html |work=How Much Sleep Do I Need? |year=2009 | author = Gavin ML, Scaivina MT}}</ref> | |||
| ====Antioxidant==== | |||
| Besides its function as synchronizer of the biological clock, melatonin is a powerful free-radical scavenger and wide-spectrum antioxidant as discovered in 1993.<ref>{{cite journal | author = Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ | title = Melatonin: a potent, endogenous hydroxyl radical scavenger | journal = Endocrine J. | year = 1993 | volume = 1 | pages = 57–60 | url = https://docs.google.com/viewer?a=v&pid=sites&srcid=ZGVmYXVsdGRvbWFpbnxkdW54aWFudGFufGd4OjVkMjA5NGZkMzFmYjRkOTU}}</ref><ref name="pmid7832450"/> In many less complex life forms, this is its only known function.<ref name="Tan_Manchester">{{cite journal | author = Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ | title = One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? | journal = J. Pineal Res. | volume = 42 | issue = 1 | pages = 28–42 | date = January 2007 | pmid = 17198536 | doi = 10.1111/j.1600-079X.2006.00407.x }}</ref> | |||
| Melatonin is an ] that can easily cross ]s<ref name = "Pohanka_2011">{{cite journal | author = Pohanka M | title = Alzheimer´s disease and related neurodegenerative disorders: implication and counteracting of melatonin | journal = Journal of Applied Biomedicine | year = 2011 | volume = 9 | pages = 185–196 | doi = 10.2478/v10136-011-0003-6|url=http://www.zsf.jcu.cz/jab/9_4/pohanka.pdf/ | issue = 4 }}</ref> | |||
| and the blood–brain barrier.<ref name="Hardeland2005"/><ref name="pmid21358970">{{cite journal | author = Reiter RJ, Manchester LC, Tan DX | title = Neurotoxins: free radical mechanisms and melatonin protection | journal = Curr Neuropharmacol | volume = 8 | issue = 3 | pages = 194–210 | date = September 2010 | pmid = 21358970 | pmc = 3001213 | doi = 10.2174/157015910792246236 }}</ref> | |||
| This antioxidant is a direct scavenger of radical oxygen and nitrogen species including OH, O<sub>2</sub><sup>−</sup>, and NO.<ref name="Melatonin Plants" /><ref name="pmid7832450">{{cite journal | author = Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR | title = Melatonin – a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro | journal = Ann. N. Y. Acad. Sci. | volume = 738 | issue = | pages = 419–20 | date = November 1994 | pmid = 7832450 | doi = 10.1111/j.1749-6632.1994.tb21831.x | bibcode = 1994NYASA.738..419P }}</ref> | |||
| Melatonin works with other antioxidants to improve the overall effectiveness of each antioxidant.<ref name="Melatonin Plants" /> Melatonin has been proven to be twice as active as vitamin E, believed to be the most effective lipophilic antioxidant.<ref name="pmid7934611">{{cite journal | author = Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F | title = Melatonin: a peroxyl radical scavenger more effective than vitamin E | journal = Life Sci. | volume = 55 | issue = 15 | pages = PL271–6 | year = 1994 | pmid = 7934611 | doi = 10.1016/0024-3205(94)00666-0 }}</ref> | |||
| An important characteristic of melatonin that distinguishes it from other classic radical scavengers is that its metabolites are also scavengers in what is referred to as the cascade reaction.<ref name="Tan_Manchester" /> Also different from other classic antioxidants, such as vitamin C and vitamin E, melatonin has amphiphilic properties. When compared to synthetic, mitochondrial-targeted antioxidants (MitoQ and MitoE), melatonin proved to be a better protector against mitochondrial oxidative stress.<ref name="pmid23381720">{{cite journal | author = Lowes DA, Webster NR, Murphy MP, Galley HF | title = Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis | journal = Br J Anaesth | volume = 110 | issue = 3 | pages = 472–80 | date = March 2013 | pmid = 23381720 | pmc = 3570068 | doi = 10.1093/bja/aes577 }}</ref> | |||
| ===Immune system=== | ===Immune system=== | ||
| Melatonin's interaction with the ] is recognized, yet the specifics of these interactions remain inadequately defined.<ref name="Carrillo-Vico2005">{{cite journal | vauthors = Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ | title = A review of the multiple actions of melatonin on the immune system | journal = Endocrine | volume = 27 | issue = 2 | pages = 189–200 | date = July 2005 | pmid = 16217132 | doi = 10.1385/ENDO:27:2:189 | s2cid = 21133107 }}</ref><ref name="Arushanian2002">{{cite journal | vauthors = Arushanian EB, Beĭer EV | title = | language = ru | journal = Eksperimental'naia i Klinicheskaia Farmakologiia | volume = 65 | issue = 5 | pages = 73–80 | year = 2002 | pmid = 12596522 }}</ref>{{Update inline|date=March 2024}} An anti-inflammatory effect appears to be the most significant.{{Citation needed|date=July 2023}} The efficacy of melatonin in disease treatment has been the subject of limited trials, with most available data deriving from small-scale, preliminary studies. It is posited that any beneficial immunological impact is attributable to melatonin's action on high-affinity receptors (MT1 and MT2), which are present on immunocompetent cells. Preclinical investigations suggest that melatonin may augment ] production and promote the expansion of ]s,<ref name="pmid16729718">{{cite journal | vauthors = Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D | title = The modulatory role of melatonin on immune responsiveness | journal = Current Opinion in Investigational Drugs | volume = 7 | issue = 5 | pages = 423–31 | date = May 2006 | pmid = 16729718 }}</ref> thereby potentially mitigating ].<ref name="Pp">{{cite journal | vauthors = Maestroni GJ | title = The immunotherapeutic potential of melatonin | journal = Expert Opinion on Investigational Drugs | volume = 10 | issue = 3 | pages = 467–76 | date = March 2001 | pmid = 11227046 | doi = 10.1517/13543784.10.3.467 | s2cid = 6822594 }}</ref> | |||
| === Weight regulation === | |||
| In ] patients, melatonin production has been found increased when compared to age-matched healthy controls.<ref name="pmid16014678">{{cite journal | author = Cutolo M, Maestroni GJ | title = The melatonin-cytokine connection in rheumatoid arthritis | journal = Ann. Rheum. Dis. | volume = 64 | issue = 8 | pages = 1109–11 | date = August 2005 | pmid = 16014678 | pmc = 1755599 | doi = 10.1136/ard.2005.038588 }}</ref>{{relevance-inline|date=April 2014}} | |||
| Melatonin's potential to regulate weight gain is posited to involve its inhibitory effect on ], a hormone that serves as a long-term indicator of the body's energy status.<ref name=":2">{{cite journal | vauthors = Suriagandhi V, Nachiappan V | title = Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance | journal = Behavioural Brain Research | volume = 417 | pages = 113598 | date = January 2022 | pmid = 34563600 | doi = 10.1016/j.bbr.2021.113598 | s2cid = 237603177 }}</ref><ref>{{cite journal | vauthors = Kelesidis T, Kelesidis I, Chou S, Mantzoros CS | title = Narrative review: the role of leptin in human physiology: emerging clinical applications | journal = Annals of Internal Medicine | volume = 152 | issue = 2 | pages = 93–100 | date = January 2010 | pmid = 20083828 | pmc = 2829242 | doi = 10.7326/0003-4819-152-2-201001190-00008 }}</ref> Leptin is important for regulating ] and body weight by signaling satiety and reducing food intake. Melatonin, by modulating leptin's actions outside of waking hours, may contribute to the restoration of leptin sensitivity during daytime, thereby counteracting ]. | |||
| ==Biochemistry== | |||
| ===Metal Chelation=== | |||
| In vitro, melatnon can form complexes with cadmium and other metals.<ref>{{cite journal|title= The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study|last= Limson|first=J.|last2= Nyokong| first2=T.| last3= Daya|first3=S.|journal= J Pineal Res|volume= 24 |year=1998|pages=15–21}}</ref> | |||
| ===Biosynthesis=== | |||
| ==Exogenous melatonin== | |||
| ] | |||
| The ] of melatonin in animals involves a sequence of ] starting with ], which can be synthesized through the ] from ], found in plants, or obtained from ]. The initial step in the melatonin biosynthesis pathway is the ] of <small>L</small>-tryptophan's ] by the enzyme ], resulting in the formation of ] (5-HTP). Subsequently, 5-HTP undergoes ], facilitated by ] and the enzyme ], yielding ].<ref>{{cite web |url=http://www.metacyc.org/META/new-image?type=PATHWAY&object=PWY-6030&detail-level=2&ENZORG=TAX-9606 |title=MetaCyc serotonin and melatonin biosynthesis }}</ref> | |||
| ===Dietary supplement=== | |||
| Serotonin, an essential ], is further converted into ] by the action of ], utilizing ].<ref name="TordjmanS">{{cite journal | vauthors = Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C | title = Melatonin: Pharmacology, Functions and Therapeutic Benefits | journal = Current Neuropharmacology | volume = 15 | issue = 3 | pages = 434–443 | date = April 2017 | pmid = 28503116 | pmc = 5405617 | doi = 10.2174/1570159X14666161228122115 }}</ref> The final step in the pathway involves the ] of ]'s ] by ], with ] as the ] donor, to produce melatonin.<ref name="TordjmanS" /> | |||
| Melatonin is categorized by the US ] (FDA) as a dietary supplement. It is sold freely over-the-counter in both the US and Canada without any regulation as a pharmaceutical drug.<ref name = "Buscemi_2004">{{cite web| author = Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassen T | title = Melatonin for treatment of sleep disorders | url = http://archive.ahrq.gov/downloads/pub/evidence/pdf/melatonin/melatonin.pdf | publisher = Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services | accessdate = 5 June 2013 |date=November 2004 | work = Evidence Report/Technology Assessment No. 108. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract No. 290-02-0023.) AHRQ Publication No. 05-E002-2. Rockville, MD: Agency for Healthcare Research and Quality }}</ref> The Food and Drug Administration (FDA) regulations applying to medications are not applicable to melatonin.<ref name="Altun2007"/> However, new FDA rules required that by June 2010 all production of dietary supplements must comply with "current ]s" (cGMP) and be manufactured with "controls that result in a consistent product free of contamination, with accurate labeling."<ref>{{cite press release | title = FDA Issues Dietary Supplements Final Rule | publisher = U.S. Food and Drug Administration | date = 2007-06-22 | url = http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108938.htm | accessdate = 2009-08-04}}</ref> The industry has also been required to report to the FDA "all serious dietary supplement related adverse events", and the FDA has (within the cGMP guidelines) begun enforcement of that requirement.<ref>{{cite web | url= http://www.medicalnewstoday.com/articles/75250.php | title = FDA Tightens Up Dietary Supplement Manufacturing And Labelling | website= Medical News Today | accessdate = 2 September 2013 | date = 26 June 2007}}</ref> | |||
| In ], ], ], and plants, the synthesis of melatonin also involves tryptophan as an intermediate but originates indirectly from the shikimate pathway. The pathway commences with <small>]</small>] and ], and in ] cells, additionally involves ]. While the subsequent biosynthetic reactions share similarities with those in animals, there are slight variations in the enzymes involved in the final stages.<ref>{{cite journal | vauthors = Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA | title = Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources | journal = Journal of Chemical Biology | volume = 5 | issue = 1 | pages = 5–17 | date = January 2012 | pmid = 22826715 | pmc = 3251648 | doi = 10.1007/s12154-011-0064-8 }}</ref><ref name="hardeland2015" /> | |||
| ===Food products=== | |||
| Melatonin has been reported in foods including cherries to about 0.17–13.46 ng/g,<ref name="pmid11600041">{{cite journal | author = Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ | title = Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus) | journal = J. Agric. Food Chem. | volume = 49 | issue = 10 | pages = 4898–902 | date = October 2001 | pmid = 11600041 | doi = 10.1021/jf010321 }}</ref> bananas and grapes, rice and cereals, herbs, olive oil, ]<ref name="pmid21342247">{{cite journal | author = Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S | title = Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection | journal = J. Pineal Res. | volume = 50 | issue = 4 | pages = 374–80 | date = May 2011 | pmid = 21342247 | doi = 10.1111/j.1600-079X.2010.00853.x }}</ref> and beer. When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains.<ref name="Hattori1995">{{cite journal | author = Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ | title = Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates | journal = Biochem. Mol. Biol. Int. | volume = 35 | issue = 3 | pages = 627–34 | date = March 1995 | pmid = 7773197 }}</ref> When humans consume foods rich in melatonin such as banana, pineapple and orange the blood levels of melatonin significantly increase.<ref name="pmid23137025">{{cite journal | author = Sae-Teaw M, Johns J, Johns NP, Subongkot S | title = Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers | journal = J. Pineal Res. | volume = 55 | issue = 1 | pages = 58–64 | date = October 2012 | pmid = 23137025 | doi = 10.1111/jpi.12025 }}</ref> | |||
| The hypothesis that melatonin synthesis occurs within mitochondria and ] suggests an evolutionary and functional significance of melatonin in cellular ] and defense mechanisms against oxidative stress, reflecting the molecule's ancient origins and its multifaceted roles across different ].<ref name="Mitochondrial biosynthesis">{{cite journal | vauthors = Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ | title = Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes | journal = Journal of Pineal Research | volume = 54 | issue = 2 | pages = 127–38 | date = March 2013 | pmid = 23137057 | doi = 10.1111/jpi.12026 | s2cid = 206140413 | doi-access = free }}</ref> | |||
| As reported in the ''New York Times'' in May 2011,<ref name="NYTimes May 2011">{{cite news |url = http://www.nytimes.com/2011/05/15/us/15lazycakes.html?_r=1&scp=1&sq=lazy%20cakes&st=cse |work=New York Times |title=Dessert, Laid-Back and Legal | date=14 May 2011 |author=Catherine Saint Louis }}</ref> beverages and snacks containing melatonin are sold in grocery stores, convenience stores, and clubs. The FDA is considering whether these food products can continue to be sold with the label "dietary supplements". On January 13, 2010, they issued a warning letter to Innovative Beverage, creators of several beverages marketed as "relaxation drinks," stating that melatonin is not approved as a ] because it is not ].<ref name="FDAwarning"/> | |||
| == |
=== Mechanism === | ||
| ] | |||
| ] | |||
| Melatonin has been studied for insomnia in the elderly.<ref name="pmid19326288">{{cite journal | author = Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, Hardeland R, Cardinali DP | title = Melatonin and melatonergic drugs on sleep: possible mechanisms of action | journal = Int. J. Neurosci. | volume = 119 | issue = 6 | pages = 821–46 | year = 2009 | pmid = 19326288 | doi = 10.1080/00207450802328607 }}</ref><ref name="pmid21358978">{{cite journal | author = Fornaro M, Prestia D, Colicchio S, Perugi G | title = A systematic, updated review on the antidepressant agomelatine focusing on its melatonergic modulation | journal = Curr Neuropharmacol | volume = 8 | issue = 3 | pages = 287–304 | date = September 2010 | pmid = 21358978 | pmc = 3001221 | doi = 10.2174/157015910792246227 }}</ref><ref name="pmid15511698">{{cite journal | author = Turek FW, Gillette MU | title = Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists | journal = Sleep Med. | volume = 5 | issue = 6 | pages = 523–32 | date = November 2004 | pmid = 15511698 | doi = 10.1016/j.sleep.2004.07.009 }}</ref> Prolonged release melatonin has shown good results in treating insomnia in older adults (2007).<ref name="pmid17875243">{{cite journal | author = Wade AG, Ford I, Crawford G, McMahon AD, Nir T, Laudon M, Zisapel N | title = Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomes | journal = Curr Med Res Opin | volume = 23 | issue = 10 | pages = 2597–605 | date = October 2007 | pmid = 17875243 | doi = 10.1185/030079907X233098 }}</ref> It may improve ] misalignment and SAD.<ref name="pmid1701579">{{cite journal | author = Cassone VM | title = Effects of melatonin on vertebrate circadian systems | journal = Trends Neurosci. | volume = 13 | issue = 11 | pages = 457–64 | date = November 1990 | pmid = 1701579 | doi = 10.1016/0166-2236(90)90099-V }}</ref><ref name="Lewy1987">{{cite journal | author = Lewy AJ, Sack RL, Miller LS, Hoban TM | title = Antidepressant and circadian phase-shifting effects of light | journal = Science | volume = 235 | issue = 4786 | pages = 352–4 | date = January 1987 | pmid = 3798117 | doi = 10.1126/science.3798117 | bibcode = 1987Sci...235..352L }}</ref> Basic research indicates that melatonin may play a role in modulating the effects of drugs of abuse such as ].<ref name="pmid10700581">{{cite journal | author = Sircar R | title = Effect of melatonin on cocaine-induced behavioral sensitization | journal = Brain Res. | volume = 857 | issue = 1–2 | pages = 295–9 | date = February 2000 | pmid = 10700581 | doi = 10.1016/S0006-8993(99)02460-9 }}</ref><ref name="Uz2003">{{cite journal | author = Uz T, Akhisaroglu M, Ahmed R, Manev H | title = The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice | journal = Neuropsychopharmacology | volume = 28 | issue = 12 | pages = 2117–23 | date = December 2003 | pmid = 12865893 | doi = 10.1038/sj.npp.1300254 }}</ref> | |||
| The mechanism of melatonin biosynthesis initiates with the hydroxylation of <small>L</small>-tryptophan, a process that requires the ] ] (THB) to react with oxygen and the active site iron of tryptophan hydroxylase. Although the complete mechanism is not entirely understood, two main mechanisms have been proposed: | |||
| ===Sleep disorders=== | |||
| The first mechanism involves a slow transfer of one ] from THB to molecular oxygen (O<sub>2</sub>), potentially producing a superoxide ({{chem2|O2-}}). This superoxide could then recombine with the THB ] to form 4a-peroxypterin. 4a-peroxypterin may either react with the active site iron (II) to create an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron, facilitating the hydroxylation of <small>L</small>-tryptophan. | |||
| A 2004 review found that "there was no evidence that melatonin had an effect on sleep onset latency or sleep efficiency" in people suffering from ], such as from shift work and rapid transmeridian travel, while it did decrease sleep onset latency in people with a primary sleep disorder and it increased sleep efficiency in people with a secondary sleep disorder.<ref name=USAHRQ /> | |||
| Alternatively, the second mechanism proposes that oxygen interacts with the active site iron (II) first, forming iron (III) superoxide. This molecule could then react with THB to form an iron-peroxypterin intermediate. | |||
| Short and long term treatment of prolonged-release melatonin was found to be effective and safe, improving sleep latency, sleep quality and daytime alertness in insomnia patients.<ref name="pmid22429105">{{cite journal | author = Lemoine P, Zisapel N | title = Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia | journal = Expert Opin Pharmacother | volume = 13 | issue = 6 | pages = 895–905 | year = 2012 | pmid = 22429105 | doi = 10.1517/14656566.2012.667076 }}</ref> | |||
| Following the formation of iron (IV) oxide from the iron-peroxypterin intermediate, this oxide selectively ] a ] to yield a ] at the C5 position of the indole ring. A subsequent ] of the hydrogen and the loss of one of the two hydrogen atoms on C5 would restore ], producing 5-hydroxy-<small>L</small>-tryptophan.<ref>{{cite journal | vauthors = Roberts KM, Fitzpatrick PF | title = Mechanisms of tryptophan and tyrosine hydroxylase | journal = IUBMB Life | volume = 65 | issue = 4 | pages = 350–7 | date = April 2013 | pmid = 23441081 | pmc = 4270200 | doi = 10.1002/iub.1144 }}</ref> | |||
| In exploratory studies, prolonged-release melatonin has shown sleep quality improvement in patients with chronic schizophrenia<ref name="pmid10847313">{{cite journal | author = Shamir E, Laudon M, Barak Y, Anis Y, Rotenberg V, Elizur A, Zisapel N | title = Melatonin improves sleep quality of patients with chronic schizophrenia | journal = J Clin Psychiatry | volume = 61 | issue = 5 | pages = 373–7 | year = 2000 | pmid = 10847313 | doi = 10.4088/jcp.v61n0509}}</ref> as well as in patients with major depressive disorder<ref name="pmid9699707">{{cite journal | author = Dolberg OT, Hirschmann S, Grunhaus L | title = Melatonin for the treatment of sleep disturbances in major depressive disorder | journal = Am J Psychiatry | volume = 155 | issue = 8 | pages = 1119–21 |date=August 1998 | pmid = 9699707 | doi = | url = http://ajp.psychiatryonline.org/article.aspx?articleid=172965 }}</ref><ref name="pmid10721684">{{cite journal | author = Dalton EJ, Rotondi D, Levitan RD, Kennedy SH, Brown GM | title = Use of slow-release melatonin in treatment-resistant depression | journal = J Psychiatry Neurosci | volume = 25 | issue = 1 | pages = 48–52 | year = 2000 | pmid = 10721684 | pmc = 1407707 | doi = }}</ref> and treating sleep-wake cycle disorders in children with underlying neurodevelopment difficulties.<ref name="pmid10949538">{{cite journal | author = Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M | title = Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders | journal = J. Pineal Res. | volume = 29 | issue = 1 | pages = 34–9 |date=August 2000 | pmid = 10949538 | doi = 10.1034/j.1600-079X.2000.290105.x }}</ref><ref name="pmid12525548">{{cite journal | author = De Leersnyder H, Bresson JL, de Blois MC, Souberbielle JC, Mogenet A, Delhotal-Landes B, Salefranque F, Munnich A | title = Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome | journal = J. Med. Genet. | volume = 40 | issue = 1 | pages = 74–8 | year = 2003 | pmid = 12525548 | pmc = 1735264 | doi = 10.1136/jmg.40.1.74}}</ref> Additionally, as add-on to antihypertensive therapy, prolonged-release melatonin improved blood pressure control in patients with nocturnal hypertension as shown in a randomised double-blind placebo controlled study.<ref name="pmid21966222">{{cite journal | author = Grossman E, Laudon M, Zisapel N | title = Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials | journal = Vasc Health Risk Manag | volume = 7 | issue = | pages = 577–84 | year = 2011 | pmid = 21966222 | pmc = 3180511 | doi = 10.2147/VHRM.S24603 }}</ref> | |||
| The decarboxylation of 5-hydroxy-<small>L</small>-tryptophan to produce 5-hydroxytryptamine is then facilitated by a decarboxylase enzyme with ] (PLP) as a cofactor.<ref>{{cite journal | vauthors = Sumi-Ichinose C, Ichinose H, Takahashi E, Hori T, Nagatsu T | title = Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis | journal = Biochemistry | volume = 31 | issue = 8 | pages = 2229–38 | date = March 1992 | pmid = 1540578 | doi = 10.1021/bi00123a004 }}</ref> PLP forms an ] with the amino acid derivative, facilitating the breaking of the ] and release of carbon dioxide. The ] of the amine derived from tryptophan restores the aromaticity of the ], leading to the production of 5-hydroxytryptamine and PLP.<ref name="ReferenceA">{{cite book | vauthors = Dewick PM | year = 2002 | title = Medicinal Natural Products. A Biosynthetic Approach | edition = 2nd | publisher = Wiley | isbn = 978-0-471-49640-3 }}</ref> | |||
| Melatonin taken in the evening is, together with ] upon awakening, the standard treatment for delayed sleep phase disorder (DSPD) and ] where circadian rhythms are not ] (biologically synchronized) to the environmental cycle. It appears to have some use against other circadian rhythm sleep disorders as well, such as ] and the problems of people who work rotating or night ]. Melatonin reduces ] to a greater extent in people with DSPD than in people with insomnia.<ref name="USAHRQ">{{cite journal | author = Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassen T | title = Melatonin for treatment of sleep disorders | journal = Evidence Report/Technology Assessment (Summary) | volume = | issue = 108 | pages = 1–7 | date = November 2004 | pmid = 15635761 | doi = | url = http://www.ncbi.nlm.nih.gov/books/NBK37431/ }}</ref> | |||
| Serotonin ''N''-acetyltransferase, with ] residue His122, is hypothesized to ] the primary amine of 5-hydroxytryptamine. This deprotonation allows the ] on the amine to attack acetyl-CoA, forming a ]. The ] from ] then acts as a ] when attacked by a general base, producing ''N''-acetylserotonin.<ref>{{cite journal | vauthors = Hickman AB, Klein DC, Dyda F | title = Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism | journal = Molecular Cell | volume = 3 | issue = 1 | pages = 23–32 | date = January 1999 | pmid = 10024876 | doi = 10.1016/S1097-2765(00)80171-9 | doi-access = free }}</ref> | |||
| The final step in the biosynthesis of melatonin involves the methylation of ''N''-acetylserotonin at the hydroxyl position by SAM, resulting in the production of ] (SAH) and melatonin.<ref name="ReferenceA" /><ref>{{cite journal | vauthors = Donohue SJ, Roseboom PH, Illnerova H, Weller JL, Klein DC | title = Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA | journal = DNA and Cell Biology | volume = 12 | issue = 8 | pages = 715–27 | date = October 1993 | pmid = 8397829 | doi = 10.1089/dna.1993.12.715 | url = https://zenodo.org/record/1235255 }}</ref> | |||
| Melatonin appears to increase the amount of sleep in people after working night shifts.<ref>{{cite journal|last1=Liira|first1=J|last2=Verbeek|first2=JH|last3=Costa|first3=G|last4=Driscoll|first4=TR|last5=Sallinen|first5=M|last6=Isotalo|first6=LK|last7=Ruotsalainen|first7=JH|title=Pharmacological interventions for sleepiness and sleep disturbances caused by shift work.|journal=The Cochrane database of systematic reviews|date=Aug 12, 2014|volume=8|pages=CD009776|pmid=25113164|doi=10.1002/14651858.CD009776.pub2}}</ref> | |||
| === Regulation === | |||
| A very small dose taken several hours before bedtime in accordance with the ] for melatonin in humans (PRC) does not cause sleepiness but, acting as a '']'' (affecting aspects of biological time structure),<ref>{{cite book | url = http://books.google.com/?id=e-bZhuzXbK4C&pg=PA433&lpg=PA433&dq=chronobiotic+definition#v=onepage&q=chronobiotic%20definition&f=false | page = 433 | chapter = Chronobiotics: Selected Agents of Potential Value in Jet Lag and other Dyschronisms | author = Simpson HW | title = Chronobiology: Principles and Application to Shifts in Schedules | editor = Sheving FE, Hagberg F | publisher = Springer | location = Berlin | year = 1979 | isbn = 978-90-286-0940-2}} The reference discusses several chronobiotic substances, but not melatonin.</ref> advances the phase slightly and is additive to the effect of using light therapy upon awakening. Light therapy may advance the phase about one to two-and-a-half hours and an oral dose of 0.3 or 3 mg of melatonin, timed correctly some hours before bedtime, can add about 30 minutes to the ~2 hour advance achieved with light therapy. There was no difference in the average magnitude of phase shift induced by the 2 doses.<ref name="pmid16295212">{{cite journal | author = Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC | title = Phase-dependent treatment of delayed sleep phase syndrome with melatonin | journal = Sleep | volume = 28 | issue = 10 | pages = 1271–8 | date = October 2005 | pmid = 16295212 | doi = }}</ref> | |||
| In vertebrates, the secretion of melatonin is regulated through the activation of the ] by the hormone ].<ref name="Nesbitt2014">{{cite journal | vauthors = Nesbitt AD, Leschziner GD, Peatfield RC | title = Headache, drugs and sleep | journal = Cephalalgia | volume = 34 | issue = 10 | pages = 756–66 | date = September 2014 | pmid = 25053748 | doi = 10.1177/0333102414542662 | s2cid = 33548757 | type = Review }}</ref> Norepinephrine increases the concentration of intracellular ] via ], which in turn activates the ] (PKA). PKA then ] ] (AANAT), the penultimate enzyme in the melatonin synthesis pathway. When exposed to daylight, noradrenergic stimulation ceases, leading to the immediate degradation of the protein by ] ].<ref>{{cite journal | vauthors = Schomerus C, Korf HW | title = Mechanisms regulating melatonin synthesis in the mammalian pineal organ | journal = Annals of the New York Academy of Sciences | volume = 1057 | issue = 1 | pages = 372–83 | date = December 2005 | pmid = 16399907 | doi = 10.1196/annals.1356.028 | bibcode = 2005NYASA1057..372S | s2cid = 20517556 }}</ref> The production of melatonin recommences in the evening, a phase known as the ]. | |||
| Blue light, especially within the {{nowrap|460–480 ]}} range, inhibits the biosynthesis of melatonin,<ref name="Brainard 2001">{{cite journal | vauthors = Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD | title = Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor | journal = The Journal of Neuroscience | volume = 21 | issue = 16 | pages = 6405–12 | date = August 2001 | pmid = 11487664 | pmc = 6763155 | doi = 10.1523/JNEUROSCI.21-16-06405.2001 }}</ref> with the degree of suppression being directly proportional to the intensity and duration of light exposure. Historically, humans in temperate climates experienced limited exposure to blue daylight during winter months, primarily receiving light from sources that emitted predominantly yellow light, such as fires.<ref>{{cite journal | vauthors = Holzman DC | title = What's in a color? The unique human health effect of blue light | journal = Environmental Health Perspectives | volume = 118 | issue = 1 | pages = A22-7 | date = January 2010 | pmid = 20061218 | pmc = 2831986 | doi = 10.1289/ehp.118-a22 }}</ref> The ] used extensively throughout the 20th century emitted relatively low levels of blue light.<ref>{{cite web|url=http://www.graphics.cornell.edu/online/measurements/source-spectra/index.html|title=Recent News – Program of Computer Graphics|website=www.graphics.cornell.edu}}</ref><!-- Kayumov ''et al.'' showed that --> It has been found that light containing only wavelengths greater than 530 nm does not suppress melatonin under bright-light conditions.<ref name="Kayumov 2005">{{cite journal | vauthors = Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM | title = Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 90 | issue = 5 | pages = 2755–61 | date = May 2005 | pmid = 15713707 | doi = 10.1210/jc.2004-2062 | doi-access = free }}</ref> The use of glasses that block blue light in the hours preceding bedtime can mitigate melatonin suppression.<ref>{{cite web|title=University of Houston study shows blue light glasses at night increase melatonin by 58%|url=https://designeroptics.com/blogs/news/university-of-houston-study-shows-blue-light-glasses-at-night-increase-melatonin-by-58|access-date=2021-08-26|website=designeroptics.com|date=25 August 2021 |language=en}}</ref> Additionally, wearing blue-blocking goggles during the last hours before bedtime is recommended for individuals needing to adjust to an earlier bedtime since melatonin facilitates the onset of sleep.<ref>{{cite journal | vauthors = Burkhart K, Phelps JR | title = Amber lenses to block blue light and improve sleep: a randomized trial | journal = Chronobiology International | volume = 26 | issue = 8 | pages = 1602–12 | date = December 2009 | pmid = 20030543 | doi = 10.3109/07420520903523719 | s2cid = 145296760 }}</ref> | |||
| ===Stimulants=== | |||
| ===Metabolism=== | |||
| Research shows that after melatonin is administered to ] patients on ], the time needed to fall asleep is significantly reduced. Furthermore, the effects of the melatonin after three months showed no change from its effects after one week of use.<ref name="Gi2003">{{cite journal | author = Tjon Pian Gi CV, Broeren JP, Starreveld JS, Versteegh FG | title = Melatonin for treatment of sleeping disorders in children with attention deficit/hyperactivity disorder: a preliminary open label study | journal = Eur. J. Pediatr. | volume = 162 | issue = 7–8 | pages = 554–5 | date = July 2003 | pmid = 12783318 | doi = 10.1007/s00431-003-1207-x }}</ref> | |||
| Melatonin is ] with an ] ranging from 20 to 50 minutes.<ref name="drugbank">{{cite web |title=Melatonin |url=https://www.drugbank.ca/drugs/DB01065 |access-date=29 January 2019 |website=www.drugbank.ca}}</ref><ref name="Auld2017" /><ref name="pmid18368944">{{cite journal |vauthors=Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP |date=2008 |title=Melatonergic drugs in clinical practice |url= |journal=Arzneimittelforschung |volume=58 |issue=1 |pages=1–10 |doi=10.1055/s-0031-1296459 |pmid=18368944 |s2cid=38857779}}</ref> The primary metabolic pathway transforms melatonin into ], which is then conjugated with sulfate and excreted in urine as a waste product.<ref name="metabolism">{{cite journal |last1=Ma |first1=Xiaochao |last2=Idle |first2=Jeffrey R. |last3=Krausz |first3=Kristopher W. |last4=Gonzalez |first4=Frank J. |title=Metabolism of Melatonin by Human Cytochromes P450 |journal=Drug Metabolism and Disposition |date=April 2005 |volume=33 |issue=4 |pages=489–494 |doi=10.1124/dmd.104.002410 |pmid=15616152 |s2cid=14555783 |url=https://dmd.aspetjournals.org/content/33/4/489 |access-date=25 January 2023}}</ref> It is primarily metabolized by the liver enzyme ] and to a lesser extent by ], ], and ].<ref name="metabolism"/> | |||
| === |
===Measurement=== | ||
| For both research and clinical purposes, melatonin levels in humans can be determined through saliva or blood plasma analysis.<ref name="pmid30919486">{{cite journal | vauthors = Kennaway DJ | title = A critical review of melatonin assays: Past and present | journal = Journal of Pineal Research | volume = 67 | issue = 1 | pages = e12572 | date = August 2019 | pmid = 30919486 | doi = 10.1111/jpi.12572 | doi-access = free }}</ref> | |||
| ==Use as a medication and supplement== | |||
| Several clinical studies indicate that supplementation with melatonin is an effective ] for ] and ]s.<ref name="Dodick2001">{{cite journal | author = Dodick DW, Capobianco DJ | title = Treatment and management of cluster headache | journal = Curr Pain Headache Rep | volume = 5 | issue = 1 | pages = 83–91 | date = February 2001 | pmid = 11252143 | doi = 10.1007/s11916-001-0015-0 }}</ref><ref name="Gagnier2001">{{cite journal | author = Gagnier JJ | title = The therapeutic potential of melatonin in migraines and other headache types | journal = Altern Med Rev | volume = 6 | issue = 4 | pages = 383–9 | date = August 2001 | pmid = 11578254 | doi = }}</ref> | |||
| {{Main|Melatonin as a medication and supplement}} | |||
| Melatonin is used both as a ] and an ] ] for the management of ]s, including ] and various ] such as ], ], and ].<ref name="pmid30148726">{{cite journal | vauthors = Riha RL | title = The use and misuse of exogenous melatonin in the treatment of sleep disorders | journal = Curr Opin Pulm Med | volume = 24 | issue = 6 | pages = 543–548 | date = November 2018 | pmid = 30148726 | doi = 10.1097/MCP.0000000000000522 | s2cid = 52096729 | url = }}</ref> In addition to melatonin, a range of synthetic ], namely ], ], and ], are used in medicine.<ref name="pmid27500861">{{cite journal | vauthors = Williams WP, McLin DE, Dressman MA, Neubauer DN | title = Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders | journal = Pharmacotherapy | volume = 36 | issue = 9 | pages = 1028–41 | date = September 2016 | pmid = 27500861 | pmc = 5108473 | doi = 10.1002/phar.1822 | url = }}</ref><ref name="pmid29487083">{{cite journal | vauthors = Atkin T, Comai S, Gobbi G |author3-link=Gabriella Gobbi| title = Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery | journal = Pharmacol Rev | volume = 70 | issue = 2 | pages = 197–245 | date = April 2018 | pmid = 29487083 | doi = 10.1124/pr.117.014381 |s2cid=3578916 | url = | doi-access = free }}</ref> | |||
| ===Cancer=== | |||
| A study published by the ] (JAMA) in April 2023 found that only 12% of the 30 melatonin gummy product preparations analyzed had melatonin quantities within ±10% of the amounts specified on their labels. Some gummy supplements were found to contain up to 347% of the declared melatonin content. In Europe, melatonin is classified as an ], highlighting the regulatory oversight of its use and distribution. Conversely, {{As of|lc=y|2022}}, the United States was considering the inclusion of melatonin in ] practices. A preceding study from 2022 concluded that consuming unregulated melatonin products can expose individuals, including children, to melatonin quantities ranging from 40 to 130 times higher than the recommended levels when products are used 'as directed'.<ref>Cohen PA, Avula B, Wang Y, Katragunta K, Khan I. (April 2023) ''JAMA''. '''329''' (16): 1401–1402. ]:10.1001/jama.2023.2296. PMID </ref> | |||
| A ] of unblinded ]s involving a total of 643 cancer patients using melatonin found a reduced incidence of death but that blinded and independently conducted randomized controlled trials are needed.<ref name="pmid16207291">{{cite journal | author = Mills E, Wu P, Seely D, Guyatt G | title = Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis | journal = J. Pineal Res. | volume = 39 | issue = 4 | pages = 360–6 | date = November 2005 | pmid = 16207291 | doi = 10.1111/j.1600-079X.2005.00258.x }}</ref> The National Cancer Institute's review of the evidence found that it remains inconclusive.<ref>{{cite web|last=National Cancer Institute|title=Topics in complementary and alternative therapies (PDQ)|url=http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0053600/|publisher=National Cancer Institute, National Institutes of Health|accessdate=5 June 2013|date=May 2013}}</ref> | |||
| Anecdotal reports and formal research studies over the past few decades have established a link between melatonin supplementation and more vivid ].<ref>{{citation |title=Effects of melatonin on dream bizarreness among male and female college students.|last=Kahan |first=Tracey L. |journal=Psychology |year=2000 |publisher=Sleep and Hypnosis, 2(2), 74-83.|url=https://scholarcommons.scu.edu/psych/102/}}</ref> | |||
| ===Gallbladder stones=== | |||
| ==History== | |||
| Melatonin presence in the gallbladder has many protective properties, such as converting cholesterol to bile, preventing oxidative stress, and increasing the mobility of gallstones from the gallbladder.<ref name="pmid18338264">{{cite journal | author = Koppisetti S, Jenigiri B, Terron MP, Tengattini S, Tamura H, Flores LJ, Tan DX, Reiter RJ | title = Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: a review | journal = Dig. Dis. Sci. | volume = 53 | issue = 10 | pages = 2592–603 | date = October 2008 | pmid = 18338264 | doi = 10.1007/s10620-007-0195-5 }}</ref> It also decreases the amount of cholesterol produced in the gallbladder by regulating the cholesterol that passes through the intestinal wall. Concentration of melatonin in the bile is 2–3 times higher than the otherwise very low daytime melatonin levels in the blood across many diurnal mammals, including humans.<ref name="pmid10622237">{{cite journal | author = Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ | title = High physiological levels of melatonin in the bile of mammals | journal = Life Sci. | volume = 65 | issue = 23 | pages = 2523–9 | date = October 1999 | pmid = 10622237 | doi = 10.1016/S0024-3205(99)00519-6 }}</ref> | |||
| {{Main|History of the pineal gland}} | |||
| === |
=== Discovery === | ||
| Melatonin's discovery is linked to the study of skin color changes in some amphibians and reptiles, a phenomenon initially observed through the administration of pineal gland extracts.<ref name="Filadelfi1996">{{cite journal | vauthors = Filadelfi AM, Castrucci AM | title = Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates | journal = Journal of Pineal Research | volume = 20 | issue = 4 | pages = 175–86 | date = May 1996 | pmid = 8836950 | doi = 10.1111/j.1600-079X.1996.tb00256.x | s2cid = 41959214 }}</ref><ref name="Sugden2004">{{cite journal | vauthors = Sugden D, Davidson K, Hough KA, Teh MT | title = Melatonin, melatonin receptors and melanophores: a moving story | journal = Pigment Cell Research | volume = 17 | issue = 5 | pages = 454–60 | date = October 2004 | pmid = 15357831 | doi = 10.1111/j.1600-0749.2004.00185.x | doi-access = free }}</ref> In 1917, Carey Pratt McCord and Floyd P. Allen found that feeding extracts from the pineal glands of cows caused the skin of tadpoles to lighten by contracting the dark ] ].<ref name="Coates">{{cite book | vauthors = Coates PM, Blackman MR, Cragg GM, Levine M, Moss J, White JD| title = Encyclopedia of dietary supplements | publisher = Marcel Dekker | location = New York, N.Y | year = 2005 | pages = 457–66 | isbn = 978-0-8247-5504-1 | url = https://books.google.com/books?id=Sfmc-fRCj10C&q=Lerner+melatonin+history&pg=PA457 }}</ref><ref name="McCord">{{cite journal |vauthors=McCord CP, Allen FP |title=Evidences associating pineal gland function with alterations in pigmentation |date=January 1917 |journal=J Exp Zool |volume= 23 |issue=1 |pages=206–24 |url=https://books.google.com/books?id=OOM1AQAAMAAJ&pg=PA207 |doi=10.1002/jez.1400230108 |bibcode=1917JEZ....23..207M }}</ref> | |||
| The hormone melatonin was isolated in 1958 by ], a ] professor, and his team at ]. Motivated by the possibility that a substance from the pineal gland could be beneficial in treating ], they extracted and identified melatonin from bovine pineal gland extracts.<ref name="pmid14415935">{{cite journal | vauthors = Lerner AB, Case JD, Takahashi Y | title = Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands | journal = The Journal of Biological Chemistry | volume = 235 | pages = 1992–7 | date = July 1960 | issue = 7 | doi = 10.1016/S0021-9258(18)69351-2 | pmid = 14415935 | doi-access = free }}</ref> Subsequent research in the mid-1970s by Lynch and others demonstrated that melatonin production follows a circadian rhythm in human pineal glands.<ref name="pmid1167425">{{cite journal | vauthors = Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH | title = Daily rhythm in human urinary melatonin | journal = Science | volume = 187 | issue = 4172 | pages = 169–71 | date = January 1975 | pmid = 1167425 | doi = 10.1126/science.1167425 | bibcode = 1975Sci...187..169L }}</ref> | |||
| Both animal<ref name="pmid10082913">{{cite journal | author = Meltz ML, Reiter RJ, Herman TS, Kumar KS | title = Melatonin and protection from whole-body irradiation: survival studies in mice | journal = Mutat. Res. | volume = 425 | issue = 1 | pages = 21–7 | date = March 1999 | pmid = 10082913 | doi = 10.1016/S0027-5107(98)00246-2 }}</ref> and human<ref name="pmid9008723">{{cite journal | author = Reiter RJ, Herman TS, Meltz ML | title = Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers | journal = Mutat. Res. | volume = 371 | issue = 3–4 | pages = 221–8 | date = December 1996 | pmid = 9008723 | doi = 10.1016/S0165-1218(96)90110-X }}</ref><ref name="pmid9541644">{{cite journal | author = Reiter RJ, Herman TS, Meltz ML | title = Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes | journal = Mutat. Res. | volume = 397 | issue = 2 | pages = 203–8 | date = February 1998 | pmid = 9541644 | doi = 10.1016/S0027-5107(97)00211-X }}</ref> studies have shown melatonin to be potentially radioprotective. Moreover, it is a more efficient protector than ],<ref name="pmid18979313">{{cite journal | author = Topkan E, Tufan H, Yavuz AA, Bacanli D, Onal C, Kosdak S, Yavuz MN | title = Comparison of the protective effects of melatonin and amifostine on radiation-induced epiphyseal injury | journal = Int. J. Radiat. Biol. | volume = 84 | issue = 10 | pages = 796–802 | date = October 2008 | pmid = 18979313 | doi = 10.1080/09553000802389678 }}</ref> a commonly used agent for this purpose. The mechanism of melatonin in protection against ionizing radiation is thought to involve scavenging of ].<ref name="Tan_Manchester"/> It is estimated that nearly 70% of biological damage caused by ionizing radiation is attributable to the free radical, especially the hydroxyl radical that attacks DNA, proteins, and cellular membranes. Melatonin has been suggested as a radioprotective agent, with the proposed advantages of being broadly protective, readily available, orally self-administered, and without major known side effects.<ref name="pmid17641465">{{cite journal | author = Shirazi A, Ghobadi G, Ghazi-Khansari M | title = A radiobiological review on melatonin: a novel radioprotector | journal = J. Radiat. Res. | volume = 48 | issue = 4 | pages = 263–72 | date = July 2007 | pmid = 17641465 | doi = 10.1269/jrr.06070 }}</ref> | |||
| The first patent for the therapeutic use of melatonin as a low-dose sleep aid was awarded to ] at the ] in 1995.<ref>{{cite patent|country=US|number=5449683|title=Methods of inducing sleep using melatonin|status=patent|fdate=16 July 1993|gdate=12 September 1995|inventor=Wurtman RJ|assign1=Massachusetts Institute of Technology}}</ref> | |||
| ===Tinnitus=== | |||
| === Etymology === | |||
| Several medical studies involving adult patients indicate that melatonin can be beneficial in the treatment of ].<ref name="pmid21859051">{{cite journal | author = Hurtuk A, Dome C, Holloman CH, Wolfe K, Welling DB, Dodson EE, Jacob A | title = Melatonin: can it stop the ringing? | journal = Ann. Otol. Rhinol. Laryngol. | volume = 120 | issue = 7 | pages = 433–40 | date = July 2011 | pmid = 21859051 | doi = }}</ref><ref name="pmid16455366">{{cite journal | author = Megwalu UC, Finnell JE, Piccirillo JF | title = The effects of melatonin on tinnitus and sleep | journal = Otolaryngol Head Neck Surg | volume = 134 | issue = 2 | pages = 210–3 | date = February 2006 | pmid = 16455366 | doi = 10.1016/j.otohns.2005.10.007 }}</ref><ref name="pmid9504599">{{cite journal | author = Rosenberg SI, Silverstein H, Rowan PT, Olds MJ | title = Effect of melatonin on tinnitus | journal = Laryngoscope | volume = 108 | issue = 3 | pages = 305–10 | date = March 1998 | pmid = 9504599 | doi = 10.1097/00005537-199803000-00001 }}</ref><ref name="pmid20207491">{{cite journal | author = Pirodda A, Raimondi MC, Ferri GG | title = Exploring the reasons why melatonin can improve tinnitus | journal = Med. Hypotheses | volume = 75 | issue = 2 | pages = 190–1 | date = August 2010 | pmid = 20207491 | doi = 10.1016/j.mehy.2010.02.018 }}</ref> | |||
| The etymology of ''melatonin'' stems from its skin-lightening properties. As detailed in their publication in the '']'',<ref name=":3">{{Cite journal |last1=Lerner |first1=Aaron B. |last2=Case |first2=James D. |last3=Takahashi |first3=Yoshiyata |last4=Lee |first4=Teh H. |last5=Mori |first5=Wataru |date=1958 |title=Isolation of melatonin, the pineal gland factor that lightens melanocytes |url=https://pubs.acs.org/doi/abs/10.1021/ja01543a060 |journal=Journal of the American Chemical Society |language=en |volume=80 |issue=10 |pages=2587 |doi=10.1021/ja01543a060 |bibcode=1958JAChS..80Q2587L |issn=0002-7863}}</ref> Lerner and his colleagues proposed the name melatonin, derived from the Greek words ''melas'', meaning 'black' or 'dark', and ''tonos'', meaning 'labour',<ref>{{Cite journal |last1=Goeser |first1=Suzanne |last2=Ruble |first2=James |last3=Chandler |first3=Linda |date=1997 |title=Melatonin: Historical and Clinical Perspectives |url=http://www.tandfonline.com/doi/full/10.1300/J088v05n01_04 |journal=Journal of Pharmaceutical Care in Pain & Symptom Control |language=en |volume=5 |issue=1 |pages=37–49 |doi=10.1300/J088v05n01_04}}</ref> 'colour'<ref>{{Cite journal |last1=Beyer |first1=C. E. |last2=Steketee |first2=J. D. |last3=Saphier |first3=D. |date=1998 |title=Antioxidant properties of melatonin–an emerging mystery |journal=Biochemical Pharmacology |volume=56 |issue=10 |pages=1265–1272 |doi=10.1016/s0006-2952(98)00180-4 |issn=0006-2952 |pmid=9825724}}</ref> or 'suppress'.<ref>{{Cite journal |last1=Liebmann |first1=P. M. |last2=Wölfler |first2=A. |last3=Felsner |first3=P. |last4=Hofer |first4=D. |last5=Schauenstein |first5=K. |date=1997 |title=Melatonin and the immune system|journal=International Archives of Allergy and Immunology |volume=112 |issue=3 |pages=203–211 |doi=10.1159/000237455 |issn=1018-2438 |pmid=9066504}}</ref> This naming convention follows that of ], another agent affecting skin color, discovered in 1948 as a modulator of ], which influenced its name based on its serum ] effect.<ref name="pmid18100415">{{cite journal |vauthors=Rapport MM, Green AA, Page IH |date=December 1948 |title=Serum vasoconstrictor, serotonin; isolation and characterization |url=http://www.jbc.org/content/176/3/1243.short |journal=The Journal of Biological Chemistry |volume=176 |issue=3 |pages=1243–1251 |doi=10.1016/S0021-9258(18)57137-4 |pmid=18100415 |doi-access=free}}</ref> Melatonin was thus aptly named to reflect its role in preventing the darkening of the skin, highlighting the intersection of biochemistry and linguistics in scientific discovery.<ref name=":3" /> | |||
| == |
==Occurrence== | ||
| Some supplemental melatonin users report an increase in vivid dreaming. Extremely high doses of melatonin (50 mg) dramatically increased ] time and dream activity in people both with and without ].<ref name=Lewis>{{cite book |title=Melatonin and the Biological Clock |last=Lewis |first=Alan |publisher=McGraw-Hill |isbn=0-87983-734-9 |year=1999 |page=23}}</ref> | |||
| === |
===Animals and Humans=== | ||
| In vertebrates, melatonin is produced in darkness, thus usually at night, by the ], a small ]<ref name="Reiter">{{cite journal | vauthors = Reiter RJ | title = Pineal melatonin: cell biology of its synthesis and of its physiological interactions | journal = Endocrine Reviews | volume = 12 | issue = 2 | pages = 151–80 | date = May 1991 | pmid = 1649044 | doi = 10.1210/edrv-12-2-151 | s2cid = 3219721 }}</ref> | |||
| located in the center of the brain but outside the ]. Light/dark information reaches the ] <!-- (SCN) --> from retinal ]s of the eyes<ref name="Richardson2005">{{cite journal | vauthors = Richardson GS | title = The human circadian system in normal and disordered sleep | journal = The Journal of Clinical Psychiatry | volume = 66 | issue = Suppl 9 | pages = 3–9; quiz 42–3 | year = 2005 | pmid = 16336035 }}</ref><ref name="Perreau-Lenz2004">{{cite journal | vauthors = Perreau-Lenz S, Pévet P, Buijs RM, Kalsbeek A | title = The biological clock: the bodyguard of temporal homeostasis | journal = Chronobiology International | volume = 21 | issue = 1 | pages = 1–25 | date = January 2004 | pmid = 15129821 | doi = 10.1081/CBI-120027984 | s2cid = 42725506 }}</ref> rather than the melatonin signal (as was once postulated). Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in ] (night-active) animals and sleep in ] ones including humans.<ref>{{cite journal | vauthors = Foster RG | title = Sleep, circadian rhythms and health | journal = Interface Focus | volume = 10 | issue = 3 | pages = 20190098 | date = June 2020 | pmid = 32382406 | pmc = 7202392 | doi = 10.1098/rsfs.2019.0098 }}</ref> | |||
| In humans, ~30 μg of melatonin is produced daily and 80% of the total amount is produced in the night (W). The plasma maximum concentration of melatonin at night are 80–120 pg/mL and the concentrations during the day are between 10–20 pg/mL.<ref>{{Cite journal |last1=Karasek |first1=M. |last2=Winczyk |first2=K. |date=2006 |title=Melatonin in humans |url=https://pubmed.ncbi.nlm.nih.gov/17218758/ |journal=Journal of Physiology and Pharmacology|volume=57 Suppl 5 |pages=19–39 |issn=1899-1505 |pmid=17218758}}</ref><ref>{{Cite journal |last1=Kolli |first1=Aditya R. |last2=Kuczaj |first2=Arkadiusz K. |last3=Calvino-Martin |first3=Florian |last4=Hoeng |first4=Julia |date=2024 |title=Simulated pharmacokinetics of inhaled caffeine and melatonin from existing products indicate the lack of dosimetric considerations |journal=Food and Chemical Toxicology |volume=187 |pages=114601 |doi=10.1016/j.fct.2024.114601 |issn=0278-6915|doi-access=free |pmid=38493979 }}</ref> | |||
| Melatonin might improve sleep in people with ]s (ASD).<ref name=asd>{{cite journal | author = Braam W, Smits MG, Didden R, Korzilius H, Van Geijlswijk IM, Curfs LM | title = Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis | journal = Dev Med Child Neurol | volume = 51 | issue = 5 | pages = 340–9 | date = May 2009 | pmid = 19379289 | doi = 10.1111/j.1469-8749.2008.03244.x | type = Meta-analysis }}</ref> | |||
| Many animals and humans use the variation in duration of melatonin production each day as a seasonal clock.<ref name="Lincoln2003">{{cite journal | vauthors = Lincoln GA, Andersson H, Loudon A | title = Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis | journal = The Journal of Endocrinology | volume = 179 | issue = 1 | pages = 1–13 | date = October 2003 | pmid = 14529560 | doi = 10.1677/joe.0.1790001 | doi-access = free }}</ref> In animals including humans,<ref name="Arendt2005">{{cite journal | vauthors = Arendt J, Skene DJ | title = Melatonin as a chronobiotic | journal = Sleep Medicine Reviews | volume = 9 | issue = 1 | pages = 25–39 | date = February 2005 | pmid = 15649736 | doi = 10.1016/j.smrv.2004.05.002 | quote = Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn), it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times. }}</ref> the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (]) seasonal functions such as reproduction, behavior, coat growth, and camouflage ] in seasonal animals.<ref name="Arendt2005" /> In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including ]<ref name="Chaturvedi">{{cite journal | pages = 803–09 | doi = 10.1071/ZO9840803 | title = Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis | year = 1984 | vauthors = Chaturvedi CM | journal = Australian Journal of Zoology | volume = 32 | issue = 6}}</ref> and hamsters.<ref name="Chen1981">{{cite journal | vauthors = Chen HJ | title = Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition | journal = Neuroendocrinology | volume = 33 | issue = 1 | pages = 43–6 | date = July 1981 | pmid = 7254478 | doi = 10.1159/000123198 }}</ref> Melatonin can suppress ] by inhibiting secretion of ] <!-- (LH) --> and ] <!-- (FSH) --> from the ] gland, especially in mammals that have a ] season when daylight hours are long. The reproduction of ] is ] and the reproduction of ] is stimulated by melatonin. In sheep, melatonin administration has also shown antioxidant and immune-modulatory regime in prenatally stressed offspring helping them survive the crucial first days of their lives.<ref>{{Cite journal |last1=Bouroutzika |first1=Efterpi |last2=Ciliberti |first2=Maria Giovanna |last3=Caroprese |first3=Mariangela |last4=Theodosiadou |first4=Ekaterini |last5=Papadopoulos |first5=Serafeim |last6=Makri |first6=Sotiria |last7=Skaperda |first7=Zoi-Vasiliki |last8=Kotsadam |first8=Georgios |last9=Michailidis |first9=Marios-Lazaros |last10=Valiakos |first10=George |last11=Chadio |first11=Stella |last12=Kouretas |first12=Dimitris |last13=Valasi |first13=Irene |date=2021-11-05 |title=Association of Melatonin Administration in Pregnant Ewes with Growth, Redox Status and Immunity of Their Offspring |journal=Animals |language=en |volume=11 |issue=11 |pages=3161 |doi=10.3390/ani11113161 |doi-access=free |issn=2076-2615 |pmc=8614450 |pmid=34827893}}</ref> | |||
| ===Pediatrics=== | |||
| During the night, melatonin regulates ], lowering its levels. | |||
| While the packaging of melatonin often warns against use in children, available studies suggest that melatonin is an efficacious and safe treatment for ADHD and sleep-onset insomnia.<ref name="pmid20028959"/> However larger and longer studies are needed to establish long-term safety and optimal dosing.<ref name="pmid20028959">{{cite journal | author = Bendz LM, Scates AC | title = Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder | journal = Ann Pharmacother | volume = 44 | issue = 1 | pages = 185–91 |date=January 2010 | pmid = 20028959 | doi = 10.1345/aph.1M365 }}</ref> | |||
| ]s have lost all the genes for melatonin synthesis as well as those for melatonin receptors.<ref name="Huelsmann2019">{{cite journal | vauthors = Huelsmann M, Hecker N, Springer MS, Gatesy J, Sharma V, Hiller M | title = Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations | journal = Science Advances | volume = 5 | issue = 9 | pages = eaaw6671 | date = September 2019 | pmid = 31579821 | pmc = 6760925 | doi = 10.1126/sciadv.aaw6671 | bibcode = 2019SciA....5.6671H }}</ref> This is thought to be related to their ] pattern (one ] at a time). Similar trends have been found in ]ns.<ref name="Huelsmann2019" /> | |||
| ==Adverse effects== | |||
| Melatonin appears to cause very few ] in the short term, up to three months, at low doses. A systematic review in 2006 showed that for sleep disorders such as ] and ], melatonin is not effective although it is safe for short term use".<ref name="pmid16423108">{{cite journal | author = Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Baker G, Klassen TP, Vohra S | title = The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis | journal = J Gen Intern Med | volume = 20 | issue = 12 | pages = 1151–8 | year = 2005 | pmid = 16423108 | pmc = 1490287 | doi = 10.1111/j.1525-1497.2005.0243.x }}</ref><ref name="Buscemi2006">{{cite journal | author = Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G | title = Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis | journal = BMJ | volume = 332 | issue = 7538 | pages = 385–93 |date=February 2006 | pmid = 16473858 | pmc = 1370968 | doi = 10.1136/bmj.38731.532766.F6 }}</ref> Prolonged-release melatonin is safe with long-term use of up to 12 months.<ref name="pmid23044640">{{cite journal | author = Lyseng-Williamson KA | title = Melatonin prolonged release: in the treatment of insomnia in patients aged ≥55 years | journal = Drugs Aging | volume = 29 | issue = 11 | pages = 911–23 | year = 2012 | pmid = 23044640 | doi = 10.1007/s40266-012-0018-z }}</ref> | |||
| ===Plants=== | |||
| Melatonin can cause nausea, next-day ], irritability,<ref>{{cite web| title=Melatonin side effects: What are the risks?| publisher=Mayo Clinic| author=Brent Bauer, M.D.| accessdate=2011-08-17| url=http://www.mayoclinic.com/health/melatonin-side-effects/AN01717}}</ref> reduced blood flow and ].<ref name="pmid11600532">{{cite journal | author = Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU | title = Melatonin treatment for age-related insomnia | journal = J. Clin. Endocrinol. Metab. | volume = 86 | issue = 10 | pages = 4727–30 | date = October 2001 | pmid = 11600532 | doi = 10.1210/jc.86.10.4727 }}</ref> Individuals with ], having reduced ] and ] to the brain when standing up, may benefit from the use of melatonin.<ref name="Chester2003">{{cite journal | author = Ray CA | title = Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans | journal = The Journal of Physiology | volume = 551 | issue = 3 | pages = 1043–8 | year = 2003 | pmid = 12869610 | pmc = 2343280 | doi = 10.1113/jphysiol.2003.043182 | laydate = September 16, 2003 | laysummary = http://www.sciencedaily.com/releases/2003/09/030916073900.htm | laysource = ScienceDaily }}</ref> | |||
| Until its identification in plants in 1987, melatonin was for decades thought to be primarily an animal neurohormone. When melatonin was identified in coffee extracts in the 1970s, it was believed to be a byproduct of the extraction process. Subsequently, however, melatonin has been found in all plants that have been investigated. It is present in all the different parts of plants, including leaves, stems, roots, fruits, and seeds, in varying proportions.<ref name="Tan_2012"/><ref>{{cite journal | vauthors = Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ | title = Phytomelatonin: a review | journal = Journal of Experimental Botany | volume = 60 | issue = 1 | pages = 57–69 | date = 1 January 2009 | pmid = 19033551 | doi = 10.1093/jxb/ern284 | s2cid = 15738948 | doi-access = free }}</ref> Melatonin concentrations differ not only among plant species, but also between varieties of the same species depending on the agronomic growing conditions, varying from picograms to several micrograms per gram.<ref name="hardeland2015">{{cite journal | vauthors = Hardeland R | title = Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions | journal = Journal of Experimental Botany | volume = 66 | issue = 3 | pages = 627–46 | date = February 2015 | pmid = 25240067 | doi = 10.1093/jxb/eru386 | doi-access = }}</ref><ref name="Bonnefont-Rousselot 2010 55–67">{{cite journal | vauthors = Bonnefont-Rousselot D, Collin F | title = Melatonin: action as antioxidant and potential applications in human disease and aging | journal = Toxicology | volume = 278 | issue = 1 | pages = 55–67 | date = November 2010 | pmid = 20417677 | doi = 10.1016/j.tox.2010.04.008 | bibcode = 2010Toxgy.278...55B }}</ref> Notably high melatonin concentrations have been measured in popular beverages such as coffee, ], ], and ], and crops including ], ], ], ], and ].<ref name="Tan_2012" /> In some common foods and beverages, including coffee<ref name="Tan_2012" /> and ],<ref>{{cite journal | vauthors = Reiter RJ, Manchester LC, Tan DX | title = Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood | journal = Nutrition | volume = 21 | issue = 9 | pages = 920–4 | date = September 2005 | pmid = 15979282 | doi = 10.1016/j.nut.2005.02.005 }}</ref> the concentration of melatonin has been estimated or measured to be sufficiently high to raise the blood level of melatonin above daytime baseline values. | |||
| In ], there is conflicting evidence whether melatonin supplementation may either ameliorate or exacerbate symptoms due to ].<ref name="Morera2001">{{cite journal | author = Morera AL, Henry M, de La Varga M | title = Seguridad en el uso de la melatonina | language = es | journal = Actas Esp Psiquiatr | volume = 29 | issue = 5 | pages = 334–7 | year = 2001 | pmid = 11602091 | doi = | trans_title = Safety in melatonin use }}</ref><ref name="Terry2008">{{cite journal | author = Terry PD, Villinger F, Bubenik GA, Sitaraman SV | title = Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research | journal = Inflamm. Bowel Dis. | volume = 15 | issue = 1 | pages = 134–40 | date = January 2009 | pmid = 18626968 | doi = 10.1002/ibd.20527 }}</ref> | |||
| Although a role for melatonin as a plant hormone has not been clearly established, its involvement in processes such as growth and photosynthesis is well established. Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described. Rather, melatonin performs important roles in plants as a growth regulator, as well as environmental stress protector. It is synthesized in plants when they are exposed to both biological stresses, for example, fungal infection, and nonbiological stresses such as extremes of temperature, toxins, increased ], drought, etc.<ref name="hardeland2015" /><ref name="reiter2015">{{cite journal | vauthors = Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A | title = Phytomelatonin: assisting plants to survive and thrive | journal = Molecules | volume = 20 | issue = 4 | pages = 7396–437 | date = April 2015 | pmid = 25911967 | pmc = 6272735 | doi = 10.3390/molecules20047396 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Arnao MB, Hernández-Ruiz J | title = Functions of melatonin in plants: a review | journal = Journal of Pineal Research | volume = 59 | issue = 2 | pages = 133–50 | date = September 2015 | pmid = 26094813 | doi = 10.1111/jpi.12253 | doi-access = free }}</ref> | |||
| Melatonin can lower ] levels.<ref name="pmid16921343">{{cite journal | author = Juszczak M, Michalska M | title = Wpływ melatoniny na syntezę i wydzielanie prolaktyny, hormonu luteinizującego (LH) i folikulotropowego (FSH) | language = pl | journal = Postepy Hig Med Dosw (Online) | volume = 60 | issue = | pages = 431–8 | year = 2006 | pmid = 16921343 | doi = | url = http://www.phmd.pl/fulltxthtml.php?ICID=453297 | trans_title = The effect of melatonin on prolactin, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) synthesis and secretion }}</ref> Effects of the hormone on human reproduction remain unclear,<ref name="pmid19905996">{{cite journal | author = Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, Brzezinski A | title = Melatonin and human reproduction: shedding light on the darkness hormone | journal = Gynecol. Endocrinol. | volume = 25 | issue = 12 | pages = 779–85 | date = December 2009 | pmid = 19905996 | doi = 10.3109/09513590903159649 }}</ref> although it was with some effect tried as a contraceptive in the 1990s.<ref>{{cite book |author=Cohen M, van Heusden AM, Verdonk HER, Wijnhamer P |chapter=Melatonin/Norethisterone contraception |editor=Touitou Y, Arendt J and Pevet P |title=Melatonin and the Pineal Gland – From Basic Science to Clinical Application |location=Amsterdam |publisher=Elsevier |year=1993 |pages=339–45 |isbn=978-0-444-89583-7}}</ref> | |||
| ]-induced ] has been experimentally mitigated ''in vivo'' in a high-melatonin ].<ref name="Park-et-al-2012">{{cite journal | vauthors = Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K | title = Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress | journal = Journal of Pineal Research | volume = 54 | issue = 3 | pages = 258–63 | date = April 2013 | pmid = 22856683 | doi = 10.1111/j.1600-079x.2012.01029.x | publisher = ] | s2cid = 6291664 }}</ref><ref name="Arnao-Hernandez-Ruiz-2014">{{cite journal | vauthors = Arnao MB, Hernández-Ruiz J | title = Melatonin: plant growth regulator and/or biostimulator during stress? | journal = Trends in Plant Science | volume = 19 | issue = 12 | pages = 789–97 | date = December 2014 | pmid = 25156541 | doi = 10.1016/j.tplants.2014.07.006 | publisher = ] | bibcode = 2014TPS....19..789A | s2cid = 38637203 }}</ref><ref name="FH">{{cite journal |first1= Hemat A. |last1= EL-Bauome | first2= Samar M. |last2= Doklega | first3= Said A. |last3= Saleh |first4= Ahmed S. |last4= Mohamed |last5= Suliman| first5= Ahmad A. | first6= Mahmoud A.M. |last6= Abd El-Hady | title = Effects of melatonin on lettuce plant growth, antioxidant enzymes and photosynthetic pigments under salinity stress conditions | journal = ] | volume = 36 | issue = 1 | pages = 1–17 | date = February 2024 | pmid = | doi = 10.2478/fhort-2024-0001 | publisher = Polish Society of Horticultural Science | s2cid = 19887642 | doi-access = free }}</ref> Studies conducted on lettuce grown in saline soil conditions have shown that the application of melatonin significantly mitigates the harmful effects of salinity. Foliar application increases the number of leaves, their surface area, increases fresh weight and the content of chlorophyll a and chlorophyll b, and the content of carotenoids compared to plants not treated with melatonin.<ref name="FH" /> | |||
| Fungal disease resistance is another role. Added melatonin increases ] in '']'' against '']''.<ref name="Arnao-Hernandez-Ruiz-2014" /><ref name="Arnao-Hernandez-Ruiz-2015">{{cite journal | vauthors = Arnao MB, Hernández-Ruiz J | title = Functions of melatonin in plants: a review | journal = Journal of Pineal Research | volume = 59 | issue = 2 | pages = 133–50 | date = September 2015 | pmid = 26094813 | doi = 10.1111/jpi.12253 | publisher = ] | s2cid = 19887642 | doi-access = free }}</ref> Also acts as a ] on ] including '']'', '']'', and '']'' spp. Decreases the speed of infection. As a ], protects '']'' from fungi. Dramatically slows ] and ].<ref name="Arnao-Hernandez-Ruiz-2015" /> | |||
| In animal models, interventions that increase the bioavailability of melatonin seem to increase the severity of the symptoms of ], whereas reduction in melatonin by pinealectomy or exposure to bright light can improve recovery from those symptoms.<ref name="pmid16596313">{{cite journal | author = Schernhammer E, Chen H, Ritz B | title = Circulating melatonin levels: possible link between Parkinson's disease and cancer risk? | journal = Cancer Causes Control. | volume = 17 | issue = 4 | pages = 577–582 | date = May 2006 | pmid = 16596313 | doi = 10.1007/s10552-005-9002-9 }}</ref> Melatonin may exacerbate neurodegeneration in advanced Parkinson's disease in rats.<ref name="pmid22252727">{{cite journal | author = Meng T, Zheng ZH, Liu TT, Lin L | title = Contralateral retinal dopamine decrease and melatonin increase in progression of hemiparkinsonium rat | journal = Neurochem Res. | volume = 37 | issue = 5 | pages = 1050–6. | date = May 2012 | pmid = 22252727 | doi = 10.1007/s11064-012-0706-4 }}</ref> | |||
| == |
===Fungi=== | ||
| Melatonin has been observed to reduce stress tolerance in '']'' in plant-pathogen systems.<ref name="Socaciu-et-al-2020">{{cite journal | vauthors = Socaciu AI, Ionuţ R, Socaciu MA, Ungur AP, Bârsan M, Chiorean A, Socaciu C, Râjnoveanu AG | display-authors = 6 | title = Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases | journal = Reviews in Endocrine & Metabolic Disorders | volume = 21 | issue = 4 | pages = 465–478 | date = December 2020 | pmid = 32691289 | doi = 10.1007/s11154-020-09570-9 | s2cid = 220657247 }}</ref> Danish pharmaceutical company ] have used genetically modified yeast ('']'') to produce melatonin.<ref>{{Cite journal |last1=Germann |first1=Susanne M. |last2=Baallal Jacobsen |first2=Simo A. |last3=Schneider |first3=Konstantin |last4=Harrison |first4=Scott J. |last5=Jensen |first5=Niels B. |last6=Chen |first6=Xiao |last7=Stahlhut |first7=Steen G. |last8=Borodina |first8=Irina |last9=Luo |first9=Hao |last10=Zhu |first10=Jiangfeng |last11=Maury |first11=Jérôme |last12=Forster |first12=Jochen |display-authors=8 |date=2016 |title=Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae |journal=Biotechnology Journal |language=en |volume=11 |issue=5 |pages=717–724 |doi=10.1002/biot.201500143 |pmc=5066760 |pmid=26710256}}</ref> | |||
| === Bacteria === | |||
| The effects of long-term supplementation of melatonin in humans have not yet been thoroughly studied nor ascertained.<ref name = "Ratzburg_Vanderbilt">{{cite web | url = http://www.vanderbilt.edu/AnS/psychology/health_psychology/melatonin.htm | title = Melatonin: The Myths and Facts | author = Ratzburg C | year = n.d. | publisher = Vanderbilt University | accessdate = 2007-12-02 }}</ref> One prescription-only, prolonged-release melatonin product, trade-name ], 2 mg, is available for up to three months use by people aged 55 and over. | |||
| Melatonin is produced by α-proteobacteria and photosynthetic cyanobacteria. There is no report of its occurrence in archaea which indicates that melatonin originated in bacteria<ref name=":1" /> most likely to prevent the first cells from the damaging effects of oxygen in the primitive Earth's atmosphere.<ref name=":0" /> | |||
| Novo Nordisk have used genetically modified '']'' to produce melatonin.<ref>{{Cite journal |last1=Luo |first1=Hao |last2=Schneider |first2=Konstantin |last3=Christensen |first3=Ulla |last4=Lei |first4=Yang |last5=Herrgard |first5=Markus |last6=Palsson |first6=Bernhard Ø. |date=2020 |title=Microbial Synthesis of Human-Hormone Melatonin at Gram Scales |url=https://pubs.acs.org/doi/10.1021/acssynbio.0c00065 |journal=ACS Synthetic Biology |language=en |volume=9 |issue=6 |pages=1240–1245 |doi=10.1021/acssynbio.0c00065 |pmid=32501000 |s2cid=219331624 |issn=2161-5063}}</ref><ref>{{Cite journal |last1=Arnao |first1=Marino B. |last2=Giraldo-Acosta |first2=Manuela |last3=Castejón-Castillejo |first3=Ana |last4=Losada-Lorán |first4=Marta |last5=Sánchez-Herrerías |first5=Pablo |last6=El Mihyaoui |first6=Amina |last7=Cano |first7=Antonio |last8=Hernández-Ruiz |first8=Josefa |date=2023 |title=Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin |journal=Metabolites |volume=13 |issue=1 |pages=72 |doi=10.3390/metabo13010072 |pmc=9862825 |pmid=36676997 |doi-access=free }}</ref> | |||
| ] pharmaceutical laboratory prepared melatonin available upon prescription.]] | |||
| === Archaea === | |||
| Immediate-release melatonin is scarcely regulated. It is available in doses from less than half a milligram to 5 mg or more. It causes blood levels of melatonin to reach their peak in about an hour. The hormone may be administered orally, as capsules, tablets or as liquid. It is also available for use sublingually, or as transdermal patches. | |||
| In 2022, the discovery of serotonin N-acetyltransferase (SNAT)'''—'''the penultimate, rate-limiting enzyme in the melatonin biosynthetic pathway'''—'''in the archaeon '']'' <ref>{{Cite journal |last1=Lee |first1=Kyungjin |last2=Choi |first2=Geun-Hee |last3=Back |first3=Kyoungwhan |date=2022-03-21 |title=Functional Characterization of Serotonin N-Acetyltransferase in Archaeon Thermoplasma volcanium |journal=Antioxidants |volume=11 |issue=3 |pages=596 |doi=10.3390/antiox11030596 |doi-access=free |issn=2076-3921 |pmc=8945778 |pmid=35326246}}</ref> firmly places melatonin biosynthesis in all three major domains of life, dating back to ~4 Gya.<ref>{{Cite journal |last1=Hoshino |first1=Yosuke |last2=Villanueva |first2=Laura |date=2023-03-10 |title=Four billion years of microbial terpenome evolution |url=https://pubmed.ncbi.nlm.nih.gov/36941124/ |journal=FEMS Microbiology Reviews |volume=47 |issue=2 |pages=fuad008 |doi=10.1093/femsre/fuad008 |issn=1574-6976 |pmid=36941124}}</ref> | |||
| === Food products === | |||
| The legal availability of melatonin varies widely among countries, ranging from being available without prescription (e.g. in most of North America and Finland) to being available only on prescription (e.g. in the European Union, Norway and Australia) or not at all (although its possession and use may not be illegal). Immediate-release melatonin is widely available on the Internet as a ]. | |||
| Naturally-occurring melatonin has been reported in foods including ] to about 0.17–13.46 ng/g,<ref name="pmid11600041">{{cite journal | vauthors = Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ | title = Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus) | journal = Journal of Agricultural and Food Chemistry | volume = 49 | issue = 10 | pages = 4898–902 | date = October 2001 | pmid = 11600041 | doi = 10.1021/jf010321 }}</ref> ], ], ], rice, ], ],<ref>{{cite journal | vauthors = González-Flores D, Velardo B, Garrido M, González-Gómez D, Lozano M, Ayuso MC, Barriga C, Paredes SD, Rodríguez AB | year = 2011 | url = https://www.researchgate.net/publication/259983119 | title = Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content | journal = Journal of Food and Nutrition Research | volume= 50 | issue = 4 | pages = 229–36 }}</ref> ], wine,<ref name="pmid21342247">{{cite journal | vauthors = Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S | title = Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection | journal = Journal of Pineal Research | volume = 50 | issue = 4 | pages = 374–80 | date = May 2011 | pmid = 21342247 | doi = 10.1111/j.1600-079X.2010.00853.x | s2cid = 8034935 }}</ref> and beer.<ref>{{cite journal|vauthors=Salehi B|date=5 July 2019|title=Melatonin in Medicinal and Food Plants|url=https://schlaf.fit/Melatonin_in_Plants_and_Food.pdf|journal=]|volume=681|access-date=2 July 2021|archive-date=29 November 2021|archive-url=https://web.archive.org/web/20211129060429/https://schlaf.fit/Melatonin_in_Plants_and_Food.pdf|url-status=dead}}</ref> The consumption of ] and sour cherries may improve sleep quality.<ref>{{cite journal | vauthors = Pereira N, Naufel MF, Ribeiro EB, Tufik S, Hachul H | title = Influence of Dietary Sources of Melatonin on Sleep Quality: A Review | journal = Journal of Food Science | volume = 85 | issue = 1 | pages = 5–13 | date = January 2020 | pmid = 31856339 | doi = 10.1111/1750-3841.14952 | publisher = Wiley | doi-access = free }}</ref> When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains.<ref name="Hattori1995">{{cite journal | vauthors = Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ | display-authors = 6 | title = Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates | journal = Biochemistry and Molecular Biology International | volume = 35 | issue = 3 | pages = 627–34 | date = March 1995 | pmid = 7773197 }}</ref> When humans consume foods rich in melatonin, such as banana, ], and ], the blood levels of melatonin increase significantly.<ref name="pmid23137025">{{cite journal | vauthors = Sae-Teaw M, Johns J, Johns NP, Subongkot S | title = Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers | journal = Journal of Pineal Research | volume = 55 | issue = 1 | pages = 58–64 | date = August 2013 | pmid = 23137025 | doi = 10.1111/jpi.12025 | s2cid = 979886 | doi-access = free }}</ref> | |||
| Formerly, melatonin was derived from animal pineal tissue, such as bovine. It is now synthetic and does not carry a risk of contamination or the means of transmitting viral material.<ref name="Altun2007"/><ref>{{cite web |url=http://www.drugs.com/melatonin.html |title=Melatonin| publisher=]| accessdate=2011-08-17}}</ref> | |||
| ===Prolonged release=== | |||
| Melatonin is available as a prolonged-release prescription drug, trade-name Circadin, manufactured by Neurim Pharmaceuticals. Containing 2 mg melatonin, it was shown in clinical trials of older adults to decrease time to fall asleep and improve quality of sleep and daytime functioning.<ref name="pmid22429105"/><ref name="pmid23044640"/> ] It releases melatonin gradually over 8–10 hours, mimicking the body's internal secretion profile. | |||
| The ] (EMA) has approved Circadin for patients aged 55 or over, as monotherapy for the short-term treatment (up to 13 weeks) of primary insomnia characterized by poor quality of sleep.<ref> Circadin (Prolonged-Release Melatonin) For Primary Insomnia Recommended For Approval In The EU (27 Apr 2007)</ref><ref name = "EMA_EPAR">{{cite web| author = European Medicines Agency | title = Circadin, melatonin | url = http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000695/human_med_000701.jsp&mid=WC0b01ac058001d124 | work = European Public Assessment Report (EPAR) | publisher = European Medicines Agency | accessdate = 5 June 2013 }}</ref> | |||
| Other countries' agencies that subsequently approved the drug include: | |||
| : --the Australian Therapeutics Goods Administration (TGA),<ref name = "TGA_APR">{{cite web | author = Therapeutic Goods Administration (TGA) | title = Australian Public Assessment Report for Melatonin | url = http://www.tga.gov.au/pdf/auspar/auspar-circadin.pdf | publisher = Department of Health and Ageing, Australian Government | accessdate = 5 June 2013 }}</ref> | |||
| : --the Swiss Agency for Therapeutics Products (SwissMedic),<ref name = "SWISSMEDIC_APR">{{cite web|author=SwissMedic|title=Circadin®, Retardtabletten, 2 mg (melatoninum)|url=https://www.swissmedic.ch/zulassungen/00153/00189/00200/01003/index.html|ref=SWISSMEDIC_APR|language=German|year=2009}}</ref> | |||
| : --the South Korean Ministry of Food and Drug Safety (MFDS)<ref name=MFDS_APP>{{cite web|title=Circadin license application|url=http://www.mfds.go.kr/jsp/common/download.jsp?fileinfo=S*2*(%C1%A4%BA%B8%B0%F8%B0%B3)_%BC%AD%C4%AB%B5%F2%BC%AD%B9%E6%C1%A42mg(%B8%E1%B6%F3%C5%E4%B4%D1)_(%BE%C8%C0%AF).pdf*bfdb417d57df46f71ca13aa03f33a890*pdf*/files/upload/1/TB_F_INFODATA/17741/bfdb417d57df46f71ca13aa03f33a890*2512375*2014:08:27%2013:58:13|publisher=MFDS|language=Korean|format=PDF|year=2014}}</ref> and | |||
| : --the Israeli Ministry of Health (MOH).<ref name=MOH_APP>{{cite web|author1=Ministry of Health Israel|title=Circadin leaflet|url=http://www.old.health.gov.il/units/pharmacy/trufot/PerutTrufa.asp?Reg_Number=139%2092%2031648%2000&safa=h}}</ref> | |||
| ==See also== | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{Reflist |
{{Reflist}} | ||
| == Further reading == | |||
| * {{cite journal | author = Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N | title = Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety | journal = BMC Med | volume = 8 | page = 51 | year = 2010 | pmid = 20712869 | pmc = 2933606 | doi = 10.1186/1741-7015-8-51 }} | |||
| == External links == | |||
| * {{Commons category inline}} | |||
| * | |||
| * | |||
| ==External links== | |||
| {{Neurotransmitters}} | |||
| {{Commons category}} | |||
| * {{cite web | url = https://www.chemwatch.net/resource-center/melatonin/ | publisher = Chemwatch | title = Melatonin }} | |||
| {{Hormones}} | {{Hormones}} | ||
| {{Antioxidants}} | {{Antioxidants}} | ||
| {{Dietary supplement}} | |||
| {{Hypnotics and sedatives}} | |||
| {{Insomnia pharmacotherapies}} | |||
| {{Melatonergics}} | |||
| {{Tryptamines}} | {{Tryptamines}} | ||
| {{Portal bar | Medicine}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:00, 22 January 2025
Hormone released by the pineal gland Not to be confused with melanin. For the album, see Melatonin (album). For the video game see Melatonin (video game) This article is about melatonin as a hormone. For its role as a supplement and medication, see Melatonin as a medication and supplement.
 | |
 | |
| Names | |
|---|---|
| IUPAC name N-acetamide | |
| Other names 5-Methoxy-N-acetyltryptamine; N-Acetyl-5-methoxytryptamine; NSC-113928 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.725 |
| EC Number |
|
| KEGG | |
| MeSH | Melatonin |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C13H16N2O2 |
| Molar mass | 232.281 g/mol |
| Melting point | 117 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Melatonin, an indoleamine, is a natural compound produced by various organisms, including bacteria and eukaryotes. Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs. This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates.
In vertebrates, melatonin's functions extend to synchronizing sleep-wake cycles, encompassing sleep-wake timing and blood pressure regulation, as well as controlling seasonal rhythmicity (circannual cycle), which includes reproduction, fattening, molting, and hibernation. Its effects are mediated through the activation of melatonin receptors and its role as an antioxidant. In plants and bacteria, melatonin primarily serves as a defense mechanism against oxidative stress, indicating its evolutionary significance. The mitochondria, key organelles within cells, are the main producers of antioxidant melatonin, underscoring the molecule's "ancient origins" and its fundamental role in protecting the earliest cells from reactive oxygen species.
In addition to its endogenous functions as a hormone and antioxidant, melatonin is also administered exogenously as a dietary supplement and medication. It is utilized in the treatment of sleep disorders, including insomnia and various circadian rhythm sleep disorders.
Biological activity
In humans, melatonin primarily acts as a potent full agonist of two types of melatonin receptors: melatonin receptor 1, with picomolar binding affinity, and melatonin receptor 2, with nanomolar binding affinity. Both receptors are part of the G-protein coupled receptors (GPCRs) family, specifically the Gi/o alpha subunit GPCRs, although melatonin receptor 1 also exhibits coupling with Gq alpha subunit.
Furthermore, melatonin functions as a high-capacity antioxidant, or free radical scavenger, within mitochondria, playing a dual role in combating cellular oxidative stress. First, it directly neutralizes free radicals, and second, it promotes the gene expression of essential antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase. This increase in antioxidant enzyme expression is mediated through signal transduction pathways activated by the binding of melatonin to its receptors. Through these mechanisms, melatonin protects the cell against oxidative stress in two ways, and plays other roles in human health than only regulating the sleep-wake cycle.
Biological functions
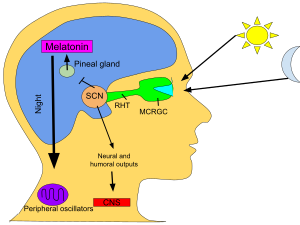
Circadian rhythm
Main article: Circadian rhythmIn mammals, melatonin is critical for the regulation of sleep–wake cycles, or circadian rhythms. The establishment of regular melatonin levels in human infants occurs around the third month after birth, with peak concentrations observed between midnight and 8:00 am. It has been documented that melatonin production diminishes as a person ages. Additionally, a shift in the timing of melatonin secretion is observed during adolescence, resulting in delayed sleep and wake times, increasing their risk for delayed sleep phase disorder during this period.
The antioxidant properties of melatonin were first recognized in 1993. In vitro studies reveal that melatonin directly neutralizes various reactive oxygen species, including hydroxyl (OH•), superoxide (O2−•), and reactive nitrogen species such as nitric oxide (NO•). In plants, melatonin works synergistically with other antioxidants, enhancing the overall effectiveness of each antioxidant. This compound has been found to be twice as efficacious as vitamin E, a known potent lipophilic antioxidant, at scavenging peroxyl radicals. The promotion of antioxidant enzyme expression, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase, is mediated through melatonin receptor-triggered signal transduction pathways.
Melatonin's concentration in the mitochondrial matrix is significantly higher than that found in the blood plasma, emphasizing its role not only in direct free radical scavenging but also in modulating the expression of antioxidant enzymes and maintaining mitochondrial integrity. This multifaceted role shows the physiological significance of melatonin as a mitochondrial antioxidant, a notion supported by numerous scholars.
Furthermore, the interaction of melatonin with reactive oxygen and nitrogen species results in the formation of metabolites capable of reducing free radicals. These metabolites, including cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK), contribute to the broader antioxidative effects of melatonin through further redox reactions with free radicals.
Immune system
Melatonin's interaction with the immune system is recognized, yet the specifics of these interactions remain inadequately defined. An anti-inflammatory effect appears to be the most significant. The efficacy of melatonin in disease treatment has been the subject of limited trials, with most available data deriving from small-scale, preliminary studies. It is posited that any beneficial immunological impact is attributable to melatonin's action on high-affinity receptors (MT1 and MT2), which are present on immunocompetent cells. Preclinical investigations suggest that melatonin may augment cytokine production and promote the expansion of T cells, thereby potentially mitigating acquired immunodeficiencies.
Weight regulation
Melatonin's potential to regulate weight gain is posited to involve its inhibitory effect on leptin, a hormone that serves as a long-term indicator of the body's energy status. Leptin is important for regulating energy balance and body weight by signaling satiety and reducing food intake. Melatonin, by modulating leptin's actions outside of waking hours, may contribute to the restoration of leptin sensitivity during daytime, thereby counteracting leptin resistance.
Biochemistry
Biosynthesis

The biosynthesis of melatonin in animals involves a sequence of enzymatic reactions starting with L-tryptophan, which can be synthesized through the shikimate pathway from chorismate, found in plants, or obtained from protein catabolism. The initial step in the melatonin biosynthesis pathway is the hydroxylation of L-tryptophan's indole ring by the enzyme tryptophan hydroxylase, resulting in the formation of 5-hydroxytryptophan (5-HTP). Subsequently, 5-HTP undergoes decarboxylation, facilitated by pyridoxal phosphate and the enzyme 5-hydroxytryptophan decarboxylase, yielding serotonin.
Serotonin, an essential neurotransmitter, is further converted into N-acetylserotonin by the action of serotonin N-acetyltransferase, utilizing acetyl-CoA. The final step in the pathway involves the methylation of N-acetylserotonin's hydroxyl group by hydroxyindole O-methyltransferase, with S-adenosyl methionine as the methyl donor, to produce melatonin.
In bacteria, protists, fungi, and plants, the synthesis of melatonin also involves tryptophan as an intermediate but originates indirectly from the shikimate pathway. The pathway commences with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells, additionally involves carbon dioxide. While the subsequent biosynthetic reactions share similarities with those in animals, there are slight variations in the enzymes involved in the final stages.
The hypothesis that melatonin synthesis occurs within mitochondria and chloroplasts suggests an evolutionary and functional significance of melatonin in cellular energy metabolism and defense mechanisms against oxidative stress, reflecting the molecule's ancient origins and its multifaceted roles across different domains of life.
Mechanism

The mechanism of melatonin biosynthesis initiates with the hydroxylation of L-tryptophan, a process that requires the cofactor tetrahydrobiopterin (THB) to react with oxygen and the active site iron of tryptophan hydroxylase. Although the complete mechanism is not entirely understood, two main mechanisms have been proposed:
The first mechanism involves a slow transfer of one electron from THB to molecular oxygen (O2), potentially producing a superoxide (O−2). This superoxide could then recombine with the THB radical to form 4a-peroxypterin. 4a-peroxypterin may either react with the active site iron (II) to create an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron, facilitating the hydroxylation of L-tryptophan.
Alternatively, the second mechanism proposes that oxygen interacts with the active site iron (II) first, forming iron (III) superoxide. This molecule could then react with THB to form an iron-peroxypterin intermediate.
Following the formation of iron (IV) oxide from the iron-peroxypterin intermediate, this oxide selectively attacks a double bond to yield a carbocation at the C5 position of the indole ring. A subsequent 1,2-shift of the hydrogen and the loss of one of the two hydrogen atoms on C5 would restore aromaticity, producing 5-hydroxy-L-tryptophan.
The decarboxylation of 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine is then facilitated by a decarboxylase enzyme with pyridoxal phosphate (PLP) as a cofactor. PLP forms an imine with the amino acid derivative, facilitating the breaking of the carbon–carbon bond and release of carbon dioxide. The protonation of the amine derived from tryptophan restores the aromaticity of the pyridine ring, leading to the production of 5-hydroxytryptamine and PLP.
Serotonin N-acetyltransferase, with histidine residue His122, is hypothesized to deprotonate the primary amine of 5-hydroxytryptamine. This deprotonation allows the lone pair on the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The thiol from coenzyme A then acts as a leaving group when attacked by a general base, producing N-acetylserotonin.
The final step in the biosynthesis of melatonin involves the methylation of N-acetylserotonin at the hydroxyl position by SAM, resulting in the production of S-adenosyl homocysteine (SAH) and melatonin.
Regulation
In vertebrates, the secretion of melatonin is regulated through the activation of the beta-1 adrenergic receptor by the hormone norepinephrine. Norepinephrine increases the concentration of intracellular cAMP via beta-adrenergic receptors, which in turn activates the cAMP-dependent protein kinase A (PKA). PKA then phosphorylates arylalkylamine N-acetyltransferase (AANAT), the penultimate enzyme in the melatonin synthesis pathway. When exposed to daylight, noradrenergic stimulation ceases, leading to the immediate degradation of the protein by proteasomal proteolysis. The production of melatonin recommences in the evening, a phase known as the dim-light melatonin onset.
Blue light, especially within the 460–480 nm range, inhibits the biosynthesis of melatonin, with the degree of suppression being directly proportional to the intensity and duration of light exposure. Historically, humans in temperate climates experienced limited exposure to blue daylight during winter months, primarily receiving light from sources that emitted predominantly yellow light, such as fires. The incandescent light bulbs used extensively throughout the 20th century emitted relatively low levels of blue light. It has been found that light containing only wavelengths greater than 530 nm does not suppress melatonin under bright-light conditions. The use of glasses that block blue light in the hours preceding bedtime can mitigate melatonin suppression. Additionally, wearing blue-blocking goggles during the last hours before bedtime is recommended for individuals needing to adjust to an earlier bedtime since melatonin facilitates the onset of sleep.
Metabolism
Melatonin is metabolized with an elimination half-life ranging from 20 to 50 minutes. The primary metabolic pathway transforms melatonin into 6-hydroxymelatonin, which is then conjugated with sulfate and excreted in urine as a waste product. It is primarily metabolized by the liver enzyme CYP1A2 and to a lesser extent by CYP1A1, CYP2C19, and CYP1B1.
Measurement
For both research and clinical purposes, melatonin levels in humans can be determined through saliva or blood plasma analysis.
Use as a medication and supplement
Main article: Melatonin as a medication and supplementMelatonin is used both as a prescription medication and an over-the-counter dietary supplement for the management of sleep disorders, including insomnia and various circadian rhythm sleep disorders such as delayed sleep phase disorder, jet lag disorder, and shift work sleep disorder. In addition to melatonin, a range of synthetic melatonin receptor agonists, namely ramelteon, tasimelteon, and agomelatine, are used in medicine.
A study published by the Journal of the American Medical Association (JAMA) in April 2023 found that only 12% of the 30 melatonin gummy product preparations analyzed had melatonin quantities within ±10% of the amounts specified on their labels. Some gummy supplements were found to contain up to 347% of the declared melatonin content. In Europe, melatonin is classified as an active pharmaceutical ingredient, highlighting the regulatory oversight of its use and distribution. Conversely, as of 2022, the United States was considering the inclusion of melatonin in pharmacy compounding practices. A preceding study from 2022 concluded that consuming unregulated melatonin products can expose individuals, including children, to melatonin quantities ranging from 40 to 130 times higher than the recommended levels when products are used 'as directed'.
Anecdotal reports and formal research studies over the past few decades have established a link between melatonin supplementation and more vivid dreams.
History
Main article: History of the pineal glandDiscovery
Melatonin's discovery is linked to the study of skin color changes in some amphibians and reptiles, a phenomenon initially observed through the administration of pineal gland extracts. In 1917, Carey Pratt McCord and Floyd P. Allen found that feeding extracts from the pineal glands of cows caused the skin of tadpoles to lighten by contracting the dark epidermal melanophores.
The hormone melatonin was isolated in 1958 by Aaron B. Lerner, a dermatology professor, and his team at Yale University. Motivated by the possibility that a substance from the pineal gland could be beneficial in treating skin diseases, they extracted and identified melatonin from bovine pineal gland extracts. Subsequent research in the mid-1970s by Lynch and others demonstrated that melatonin production follows a circadian rhythm in human pineal glands.
The first patent for the therapeutic use of melatonin as a low-dose sleep aid was awarded to Richard Wurtman at the Massachusetts Institute of Technology in 1995.
Etymology
The etymology of melatonin stems from its skin-lightening properties. As detailed in their publication in the Journal of the American Chemical Society, Lerner and his colleagues proposed the name melatonin, derived from the Greek words melas, meaning 'black' or 'dark', and tonos, meaning 'labour', 'colour' or 'suppress'. This naming convention follows that of serotonin, another agent affecting skin color, discovered in 1948 as a modulator of vascular tone, which influenced its name based on its serum vasoconstrictor effect. Melatonin was thus aptly named to reflect its role in preventing the darkening of the skin, highlighting the intersection of biochemistry and linguistics in scientific discovery.
Occurrence
Animals and Humans
In vertebrates, melatonin is produced in darkness, thus usually at night, by the pineal gland, a small endocrine gland located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the suprachiasmatic nuclei from retinal photosensitive ganglion cells of the eyes rather than the melatonin signal (as was once postulated). Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.
In humans, ~30 μg of melatonin is produced daily and 80% of the total amount is produced in the night (W). The plasma maximum concentration of melatonin at night are 80–120 pg/mL and the concentrations during the day are between 10–20 pg/mL.
Many animals and humans use the variation in duration of melatonin production each day as a seasonal clock. In animals including humans, the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage coloring in seasonal animals. In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including mynah birds and hamsters. Melatonin can suppress libido by inhibiting secretion of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is stimulated by melatonin. In sheep, melatonin administration has also shown antioxidant and immune-modulatory regime in prenatally stressed offspring helping them survive the crucial first days of their lives.
During the night, melatonin regulates leptin, lowering its levels.
Cetaceans have lost all the genes for melatonin synthesis as well as those for melatonin receptors. This is thought to be related to their unihemispheric sleep pattern (one brain hemisphere at a time). Similar trends have been found in sirenians.
Plants
Until its identification in plants in 1987, melatonin was for decades thought to be primarily an animal neurohormone. When melatonin was identified in coffee extracts in the 1970s, it was believed to be a byproduct of the extraction process. Subsequently, however, melatonin has been found in all plants that have been investigated. It is present in all the different parts of plants, including leaves, stems, roots, fruits, and seeds, in varying proportions. Melatonin concentrations differ not only among plant species, but also between varieties of the same species depending on the agronomic growing conditions, varying from picograms to several micrograms per gram. Notably high melatonin concentrations have been measured in popular beverages such as coffee, tea, wine, and beer, and crops including corn, rice, wheat, barley, and oats. In some common foods and beverages, including coffee and walnuts, the concentration of melatonin has been estimated or measured to be sufficiently high to raise the blood level of melatonin above daytime baseline values.
Although a role for melatonin as a plant hormone has not been clearly established, its involvement in processes such as growth and photosynthesis is well established. Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described. Rather, melatonin performs important roles in plants as a growth regulator, as well as environmental stress protector. It is synthesized in plants when they are exposed to both biological stresses, for example, fungal infection, and nonbiological stresses such as extremes of temperature, toxins, increased soil salinity, drought, etc.
Herbicide-induced oxidative stress has been experimentally mitigated in vivo in a high-melatonin transgenic rice. Studies conducted on lettuce grown in saline soil conditions have shown that the application of melatonin significantly mitigates the harmful effects of salinity. Foliar application increases the number of leaves, their surface area, increases fresh weight and the content of chlorophyll a and chlorophyll b, and the content of carotenoids compared to plants not treated with melatonin.
Fungal disease resistance is another role. Added melatonin increases resistance in Malus prunifolia against Diplocarpon mali. Also acts as a growth inhibitor on fungal pathogens including Alternaria, Botrytis, and Fusarium spp. Decreases the speed of infection. As a seed treatment, protects Lupinus albus from fungi. Dramatically slows Pseudomonas syringae tomato DC3000 infecting Arabidopsis thaliana and infecting Nicotiana benthamiana.
Fungi
Melatonin has been observed to reduce stress tolerance in Phytophthora infestans in plant-pathogen systems. Danish pharmaceutical company Novo Nordisk have used genetically modified yeast (Saccharomyces cerevisiae) to produce melatonin.
Bacteria
Melatonin is produced by α-proteobacteria and photosynthetic cyanobacteria. There is no report of its occurrence in archaea which indicates that melatonin originated in bacteria most likely to prevent the first cells from the damaging effects of oxygen in the primitive Earth's atmosphere.
Novo Nordisk have used genetically modified Escherichia coli to produce melatonin.
Archaea
In 2022, the discovery of serotonin N-acetyltransferase (SNAT)—the penultimate, rate-limiting enzyme in the melatonin biosynthetic pathway—in the archaeon Thermoplasma volcanium firmly places melatonin biosynthesis in all three major domains of life, dating back to ~4 Gya.
Food products
Naturally-occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g, bananas, plums, grapes, rice, cereals, herbs, olive oil, wine, and beer. The consumption of milk and sour cherries may improve sleep quality. When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains. When humans consume foods rich in melatonin, such as banana, pineapple, and orange, the blood levels of melatonin increase significantly.
References
- Amaral FG, Cipolla-Neto J (2018). "A brief review about melatonin, a pineal hormone". Archives of Endocrinology and Metabolism. 62 (4): 472–479. doi:10.20945/2359-3997000000066. PMC 10118741. PMID 30304113. S2CID 52954755.
- ^ Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL (August 2017). "Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders" (PDF). Sleep Medicine Reviews. 34: 10–22. doi:10.1016/j.smrv.2016.06.005. hdl:20.500.11820/0e890bda-4b1d-4786-a907-a03b1580fd07. PMID 28648359.
- Faraone SV (2014). ADHD: Non-Pharmacologic Interventions, An Issue of Child and Adolescent Psychiatric Clinics of North America, E-Book. Elsevier Health Sciences. p. 888. ISBN 978-0-323-32602-5.
- Altun A, Ugur-Altun B (May 2007). "Melatonin: therapeutic and clinical utilization". International Journal of Clinical Practice. 61 (5): 835–45. doi:10.1111/j.1742-1241.2006.01191.x. PMID 17298593. S2CID 18050554.
- Boutin JA, Audinot V, Ferry G, Delagrange P (August 2005). "Molecular tools to study melatonin pathways and actions". Trends in Pharmacological Sciences. 26 (8): 412–9. doi:10.1016/j.tips.2005.06.006. PMID 15992934.
- Hardeland R (July 2005). "Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance". Endocrine. 27 (2): 119–30. doi:10.1385/ENDO:27:2:119. PMID 16217125. S2CID 46984486.
- Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (June 2001). "Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system". Annals of the New York Academy of Sciences. 939 (1): 200–15. Bibcode:2001NYASA.939..200R. doi:10.1111/j.1749-6632.2001.tb03627.x. PMID 11462772. S2CID 20404509.
- ^ Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (January 2012). "Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science". Journal of Experimental Botany. 63 (2): 577–97. doi:10.1093/jxb/err256. PMID 22016420.
- Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018). "Mitochondria: Central Organelles for Melatonin's Antioxidant and Anti-Aging Actions". Molecules. 23 (2): 509. doi:10.3390/molecules23020509. PMC 6017324. PMID 29495303.
- ^ Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015). "Melatonin: an ancient molecule that makes oxygen metabolically tolerable". Journal of Pineal Research. 59 (4): 403–419. doi:10.1111/jpi.12267. PMID 26272235. S2CID 24373303.
- ^ Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019). "Melatonin Synthesis and Function: Evolutionary History in Animals and Plants". Frontiers in Endocrinology. 10: 249. doi:10.3389/fendo.2019.00249. PMC 6481276. PMID 31057485.
- ^ Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. (September 2016). "Update on melatonin receptors: IUPHAR Review 20". British Journal of Pharmacology. 173 (18): 2702–25. doi:10.1111/bph.13536. PMC 4995287. PMID 27314810.
Hence, one melatonin molecule and its associated metabolites could scavenge a large number of reactive species, and thus, the overall antioxidant capacity of melatonin is believed to be greater than that of other well-known antioxidants, such as vitamin C and vitamin E, under in vitro or in vivo conditions (Gitto et al., 2001; Sharma and Haldar, 2006; Ortiz et al., 2013).
- "Melatonin receptors | G protein-coupled receptors | IUPHAR/BPS Guide to Pharmacology". www.guidetopharmacology.org. Retrieved 7 April 2017.
- ^ Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A (2017). "Melatonin and human mitochondrial diseases". Journal of Research in Medical Sciences. 22: 2. doi:10.4103/1735-1995.199092. PMC 5361446. PMID 28400824.
- ^ Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (November 2017). "Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas". Cellular and Molecular Life Sciences. 74 (21): 3863–3881. doi:10.1007/s00018-017-2609-7. PMC 11107735. PMID 28864909. S2CID 23820389.
melatonin is specifically targeted to the mitochondria where it seems to function as an apex antioxidant ... The measurement of the subcellular distribution of melatonin has shown that the concentration of this indole in the mitochondria greatly exceeds that in the blood.
- ^ Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (October 2016). "Melatonin as an antioxidant: under promises but over delivers". Journal of Pineal Research. 61 (3): 253–78. doi:10.1111/jpi.12360. PMID 27500468. S2CID 35435683.
There is credible evidence to suggest that melatonin should be classified as a mitochondria-targeted antioxidant.
- ^ Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, et al. (November 2015). "Melatonin: an ancient molecule that makes oxygen metabolically tolerable". Journal of Pineal Research. 59 (4): 403–19. doi:10.1111/jpi.12267. PMID 26272235. S2CID 24373303.
While originally thought to be produced exclusively in and secreted from the vertebrate pineal gland , it is now known that the indole is present in many, perhaps all, vertebrate organs and in organs of all plants that have been investigated . That melatonin is not relegated solely to the pineal gland is also emphasized by the reports that it is present in invertebrates , which lack a pineal gland and some of which consist of only a single cell.
- ^ Mayo JC, Sainz RM, González-Menéndez P, Hevia D, Cernuda-Cernuda R (November 2017). "Melatonin transport into mitochondria". Cellular and Molecular Life Sciences. 74 (21): 3927–3940. doi:10.1007/s00018-017-2616-8. PMC 11107582. PMID 28828619. S2CID 10920415.
- Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A (June 2016). "A Review of Melatonin, Its Receptors and Drugs". The Eurasian Journal of Medicine. 48 (2): 135–41. doi:10.5152/eurasianjmed.2015.0267. PMC 4970552. PMID 27551178.
- Ardura J, Gutierrez R, Andres J, Agapito T (2003). "Emergence and evolution of the circadian rhythm of melatonin in children". Hormone Research. 59 (2): 66–72. doi:10.1159/000068571 (inactive 10 December 2024). PMID 12589109. S2CID 41937922.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM (1986). "Human melatonin production decreases with age". Journal of Pineal Research. 3 (4): 379–88. doi:10.1111/j.1600-079X.1986.tb00760.x. PMID 3783419. S2CID 33664568.
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA (June 2009). "Adolescent changes in the homeostatic and circadian regulation of sleep". Developmental Neuroscience. 31 (4): 276–84. doi:10.1159/000216538. PMC 2820578. PMID 19546564.
- Tan DX, Chen LD, Poeggeler B, L Manchester C, Reiter RJ (1993). "Melatonin: a potent, endogenous hydroxyl radical scavenger". Endocr. J. 1: 57–60.
- Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR (November 1994). "Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro". Annals of the New York Academy of Sciences. 738 (1): 419–20. Bibcode:1994NYASA.738..419P. doi:10.1111/j.1749-6632.1994.tb21831.x. PMID 7832450. S2CID 36383425.
- ^ Arnao MB, Hernández-Ruiz J (May 2006). "The physiological function of melatonin in plants". Plant Signaling & Behavior. 1 (3): 89–95. Bibcode:2006PlSiB...1...89A. doi:10.4161/psb.1.3.2640. PMC 2635004. PMID 19521488.
- Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994). "Melatonin: a peroxyl radical scavenger more effective than vitamin E". Life Sciences. 55 (15): PL271-6. doi:10.1016/0024-3205(94)00666-0. PMID 7934611.
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ (July 2005). "A review of the multiple actions of melatonin on the immune system". Endocrine. 27 (2): 189–200. doi:10.1385/ENDO:27:2:189. PMID 16217132. S2CID 21133107.
- Arushanian EB, Beĭer EV (2002). "". Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 65 (5): 73–80. PMID 12596522.
- Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (May 2006). "The modulatory role of melatonin on immune responsiveness". Current Opinion in Investigational Drugs. 7 (5): 423–31. PMID 16729718.
- Maestroni GJ (March 2001). "The immunotherapeutic potential of melatonin". Expert Opinion on Investigational Drugs. 10 (3): 467–76. doi:10.1517/13543784.10.3.467. PMID 11227046. S2CID 6822594.
- Suriagandhi V, Nachiappan V (January 2022). "Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance". Behavioural Brain Research. 417: 113598. doi:10.1016/j.bbr.2021.113598. PMID 34563600. S2CID 237603177.
- Kelesidis T, Kelesidis I, Chou S, Mantzoros CS (January 2010). "Narrative review: the role of leptin in human physiology: emerging clinical applications". Annals of Internal Medicine. 152 (2): 93–100. doi:10.7326/0003-4819-152-2-201001190-00008. PMC 2829242. PMID 20083828.
- "MetaCyc serotonin and melatonin biosynthesis".
- ^ Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C (April 2017). "Melatonin: Pharmacology, Functions and Therapeutic Benefits". Current Neuropharmacology. 15 (3): 434–443. doi:10.2174/1570159X14666161228122115. PMC 5405617. PMID 28503116.
- Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (January 2012). "Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources". Journal of Chemical Biology. 5 (1): 5–17. doi:10.1007/s12154-011-0064-8. PMC 3251648. PMID 22826715.
- ^ Hardeland R (February 2015). "Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions". Journal of Experimental Botany. 66 (3): 627–46. doi:10.1093/jxb/eru386. PMID 25240067.
- Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (March 2013). "Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes". Journal of Pineal Research. 54 (2): 127–38. doi:10.1111/jpi.12026. PMID 23137057. S2CID 206140413.
- Roberts KM, Fitzpatrick PF (April 2013). "Mechanisms of tryptophan and tyrosine hydroxylase". IUBMB Life. 65 (4): 350–7. doi:10.1002/iub.1144. PMC 4270200. PMID 23441081.
- Sumi-Ichinose C, Ichinose H, Takahashi E, Hori T, Nagatsu T (March 1992). "Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis". Biochemistry. 31 (8): 2229–38. doi:10.1021/bi00123a004. PMID 1540578.
- ^ Dewick PM (2002). Medicinal Natural Products. A Biosynthetic Approach (2nd ed.). Wiley. ISBN 978-0-471-49640-3.
- Hickman AB, Klein DC, Dyda F (January 1999). "Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism". Molecular Cell. 3 (1): 23–32. doi:10.1016/S1097-2765(00)80171-9. PMID 10024876.
- Donohue SJ, Roseboom PH, Illnerova H, Weller JL, Klein DC (October 1993). "Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA". DNA and Cell Biology. 12 (8): 715–27. doi:10.1089/dna.1993.12.715. PMID 8397829.
- Nesbitt AD, Leschziner GD, Peatfield RC (September 2014). "Headache, drugs and sleep". Cephalalgia (Review). 34 (10): 756–66. doi:10.1177/0333102414542662. PMID 25053748. S2CID 33548757.
- Schomerus C, Korf HW (December 2005). "Mechanisms regulating melatonin synthesis in the mammalian pineal organ". Annals of the New York Academy of Sciences. 1057 (1): 372–83. Bibcode:2005NYASA1057..372S. doi:10.1196/annals.1356.028. PMID 16399907. S2CID 20517556.
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD (August 2001). "Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor". The Journal of Neuroscience. 21 (16): 6405–12. doi:10.1523/JNEUROSCI.21-16-06405.2001. PMC 6763155. PMID 11487664.
- Holzman DC (January 2010). "What's in a color? The unique human health effect of blue light". Environmental Health Perspectives. 118 (1): A22-7. doi:10.1289/ehp.118-a22. PMC 2831986. PMID 20061218.
- "Recent News – Program of Computer Graphics". www.graphics.cornell.edu.
- Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM (May 2005). "Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work". The Journal of Clinical Endocrinology and Metabolism. 90 (5): 2755–61. doi:10.1210/jc.2004-2062. PMID 15713707.
- "University of Houston study shows blue light glasses at night increase melatonin by 58%". designeroptics.com. 25 August 2021. Retrieved 26 August 2021.
- Burkhart K, Phelps JR (December 2009). "Amber lenses to block blue light and improve sleep: a randomized trial". Chronobiology International. 26 (8): 1602–12. doi:10.3109/07420520903523719. PMID 20030543. S2CID 145296760.
- "Melatonin". www.drugbank.ca. Retrieved 29 January 2019.
- Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP (2008). "Melatonergic drugs in clinical practice". Arzneimittelforschung. 58 (1): 1–10. doi:10.1055/s-0031-1296459. PMID 18368944. S2CID 38857779.
- ^ Ma X, Idle JR, Krausz KW, Gonzalez FJ (April 2005). "Metabolism of Melatonin by Human Cytochromes P450". Drug Metabolism and Disposition. 33 (4): 489–494. doi:10.1124/dmd.104.002410. PMID 15616152. S2CID 14555783. Retrieved 25 January 2023.
- Kennaway DJ (August 2019). "A critical review of melatonin assays: Past and present". Journal of Pineal Research. 67 (1): e12572. doi:10.1111/jpi.12572. PMID 30919486.
- Riha RL (November 2018). "The use and misuse of exogenous melatonin in the treatment of sleep disorders". Curr Opin Pulm Med. 24 (6): 543–548. doi:10.1097/MCP.0000000000000522. PMID 30148726. S2CID 52096729.
- Williams WP, McLin DE, Dressman MA, Neubauer DN (September 2016). "Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders". Pharmacotherapy. 36 (9): 1028–41. doi:10.1002/phar.1822. PMC 5108473. PMID 27500861.
- Atkin T, Comai S, Gobbi G (April 2018). "Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery". Pharmacol Rev. 70 (2): 197–245. doi:10.1124/pr.117.014381. PMID 29487083. S2CID 3578916.
- Cohen PA, Avula B, Wang Y, Katragunta K, Khan I. (April 2023) "Quantity of Melatonin and CBD in Melatonin Gummies Sold in the US" JAMA. 329 (16): 1401–1402. doi:10.1001/jama.2023.2296. PMID 37097362
- Kahan TL (2000), "Effects of melatonin on dream bizarreness among male and female college students.", Psychology, Sleep and Hypnosis, 2(2), 74-83.
- Filadelfi AM, Castrucci AM (May 1996). "Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates". Journal of Pineal Research. 20 (4): 175–86. doi:10.1111/j.1600-079X.1996.tb00256.x. PMID 8836950. S2CID 41959214.
- Sugden D, Davidson K, Hough KA, Teh MT (October 2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Research. 17 (5): 454–60. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- Coates PM, Blackman MR, Cragg GM, Levine M, Moss J, White JD (2005). Encyclopedia of dietary supplements. New York, N.Y: Marcel Dekker. pp. 457–66. ISBN 978-0-8247-5504-1.
- McCord CP, Allen FP (January 1917). "Evidences associating pineal gland function with alterations in pigmentation". J Exp Zool. 23 (1): 206–24. Bibcode:1917JEZ....23..207M. doi:10.1002/jez.1400230108.
- Lerner AB, Case JD, Takahashi Y (July 1960). "Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands". The Journal of Biological Chemistry. 235 (7): 1992–7. doi:10.1016/S0021-9258(18)69351-2. PMID 14415935.
- Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH (January 1975). "Daily rhythm in human urinary melatonin". Science. 187 (4172): 169–71. Bibcode:1975Sci...187..169L. doi:10.1126/science.1167425. PMID 1167425.
- US patent 5449683, Wurtman RJ, "Methods of inducing sleep using melatonin", issued 12 September 1995, assigned to Massachusetts Institute of Technology
- ^ Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958). "Isolation of melatonin, the pineal gland factor that lightens melanocytes". Journal of the American Chemical Society. 80 (10): 2587. Bibcode:1958JAChS..80Q2587L. doi:10.1021/ja01543a060. ISSN 0002-7863.
- Goeser S, Ruble J, Chandler L (1997). "Melatonin: Historical and Clinical Perspectives". Journal of Pharmaceutical Care in Pain & Symptom Control. 5 (1): 37–49. doi:10.1300/J088v05n01_04.
- Beyer CE, Steketee JD, Saphier D (1998). "Antioxidant properties of melatonin–an emerging mystery". Biochemical Pharmacology. 56 (10): 1265–1272. doi:10.1016/s0006-2952(98)00180-4. ISSN 0006-2952. PMID 9825724.
- Liebmann PM, Wölfler A, Felsner P, Hofer D, Schauenstein K (1997). "Melatonin and the immune system". International Archives of Allergy and Immunology. 112 (3): 203–211. doi:10.1159/000237455. ISSN 1018-2438. PMID 9066504.
- Rapport MM, Green AA, Page IH (December 1948). "Serum vasoconstrictor, serotonin; isolation and characterization". The Journal of Biological Chemistry. 176 (3): 1243–1251. doi:10.1016/S0021-9258(18)57137-4. PMID 18100415.
- Reiter RJ (May 1991). "Pineal melatonin: cell biology of its synthesis and of its physiological interactions". Endocrine Reviews. 12 (2): 151–80. doi:10.1210/edrv-12-2-151. PMID 1649044. S2CID 3219721.
- Richardson GS (2005). "The human circadian system in normal and disordered sleep". The Journal of Clinical Psychiatry. 66 (Suppl 9): 3–9, quiz 42–3. PMID 16336035.
- Perreau-Lenz S, Pévet P, Buijs RM, Kalsbeek A (January 2004). "The biological clock: the bodyguard of temporal homeostasis". Chronobiology International. 21 (1): 1–25. doi:10.1081/CBI-120027984. PMID 15129821. S2CID 42725506.
- Foster RG (June 2020). "Sleep, circadian rhythms and health". Interface Focus. 10 (3): 20190098. doi:10.1098/rsfs.2019.0098. PMC 7202392. PMID 32382406.
- Karasek M, Winczyk K (2006). "Melatonin in humans". Journal of Physiology and Pharmacology. 57 Suppl 5: 19–39. ISSN 1899-1505. PMID 17218758.
- Kolli AR, Kuczaj AK, Calvino-Martin F, Hoeng J (2024). "Simulated pharmacokinetics of inhaled caffeine and melatonin from existing products indicate the lack of dosimetric considerations". Food and Chemical Toxicology. 187: 114601. doi:10.1016/j.fct.2024.114601. ISSN 0278-6915. PMID 38493979.
- Lincoln GA, Andersson H, Loudon A (October 2003). "Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis". The Journal of Endocrinology. 179 (1): 1–13. doi:10.1677/joe.0.1790001. PMID 14529560.
- ^ Arendt J, Skene DJ (February 2005). "Melatonin as a chronobiotic". Sleep Medicine Reviews. 9 (1): 25–39. doi:10.1016/j.smrv.2004.05.002. PMID 15649736.
Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn), it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times.
- Chaturvedi CM (1984). "Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis". Australian Journal of Zoology. 32 (6): 803–09. doi:10.1071/ZO9840803.
- Chen HJ (July 1981). "Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition". Neuroendocrinology. 33 (1): 43–6. doi:10.1159/000123198. PMID 7254478.
- Bouroutzika E, Ciliberti MG, Caroprese M, Theodosiadou E, Papadopoulos S, Makri S, Skaperda ZV, Kotsadam G, Michailidis ML, Valiakos G, Chadio S, Kouretas D, Valasi I (5 November 2021). "Association of Melatonin Administration in Pregnant Ewes with Growth, Redox Status and Immunity of Their Offspring". Animals. 11 (11): 3161. doi:10.3390/ani11113161. ISSN 2076-2615. PMC 8614450. PMID 34827893.
- ^ Huelsmann M, Hecker N, Springer MS, Gatesy J, Sharma V, Hiller M (September 2019). "Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations". Science Advances. 5 (9): eaaw6671. Bibcode:2019SciA....5.6671H. doi:10.1126/sciadv.aaw6671. PMC 6760925. PMID 31579821.
- Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (1 January 2009). "Phytomelatonin: a review". Journal of Experimental Botany. 60 (1): 57–69. doi:10.1093/jxb/ern284. PMID 19033551. S2CID 15738948.
- Bonnefont-Rousselot D, Collin F (November 2010). "Melatonin: action as antioxidant and potential applications in human disease and aging". Toxicology. 278 (1): 55–67. Bibcode:2010Toxgy.278...55B. doi:10.1016/j.tox.2010.04.008. PMID 20417677.
- Reiter RJ, Manchester LC, Tan DX (September 2005). "Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood". Nutrition. 21 (9): 920–4. doi:10.1016/j.nut.2005.02.005. PMID 15979282.
- Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A (April 2015). "Phytomelatonin: assisting plants to survive and thrive". Molecules. 20 (4): 7396–437. doi:10.3390/molecules20047396. PMC 6272735. PMID 25911967.
- Arnao MB, Hernández-Ruiz J (September 2015). "Functions of melatonin in plants: a review". Journal of Pineal Research. 59 (2): 133–50. doi:10.1111/jpi.12253. PMID 26094813.
- Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (April 2013). "Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress". Journal of Pineal Research. 54 (3). Wiley: 258–63. doi:10.1111/j.1600-079x.2012.01029.x. PMID 22856683. S2CID 6291664.
- ^ Arnao MB, Hernández-Ruiz J (December 2014). "Melatonin: plant growth regulator and/or biostimulator during stress?". Trends in Plant Science. 19 (12). Elsevier: 789–97. Bibcode:2014TPS....19..789A. doi:10.1016/j.tplants.2014.07.006. PMID 25156541. S2CID 38637203.
- ^ EL-Bauome HA, Doklega SM, Saleh SA, Mohamed AS, Suliman AA, Abd El-Hady MA (February 2024). "Effects of melatonin on lettuce plant growth, antioxidant enzymes and photosynthetic pigments under salinity stress conditions". Folia Horticulturae. 36 (1). Polish Society of Horticultural Science: 1–17. doi:10.2478/fhort-2024-0001. S2CID 19887642.
- ^ Arnao MB, Hernández-Ruiz J (September 2015). "Functions of melatonin in plants: a review". Journal of Pineal Research. 59 (2). Wiley: 133–50. doi:10.1111/jpi.12253. PMID 26094813. S2CID 19887642.
- Socaciu AI, Ionuţ R, Socaciu MA, Ungur AP, Bârsan M, Chiorean A, et al. (December 2020). "Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases". Reviews in Endocrine & Metabolic Disorders. 21 (4): 465–478. doi:10.1007/s11154-020-09570-9. PMID 32691289. S2CID 220657247.
- Germann SM, Baallal Jacobsen SA, Schneider K, Harrison SJ, Jensen NB, Chen X, Stahlhut SG, Borodina I, et al. (2016). "Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae". Biotechnology Journal. 11 (5): 717–724. doi:10.1002/biot.201500143. PMC 5066760. PMID 26710256.
- Luo H, Schneider K, Christensen U, Lei Y, Herrgard M, Palsson BØ (2020). "Microbial Synthesis of Human-Hormone Melatonin at Gram Scales". ACS Synthetic Biology. 9 (6): 1240–1245. doi:10.1021/acssynbio.0c00065. ISSN 2161-5063. PMID 32501000. S2CID 219331624.
- Arnao MB, Giraldo-Acosta M, Castejón-Castillejo A, Losada-Lorán M, Sánchez-Herrerías P, El Mihyaoui A, Cano A, Hernández-Ruiz J (2023). "Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin". Metabolites. 13 (1): 72. doi:10.3390/metabo13010072. PMC 9862825. PMID 36676997.
- Lee K, Choi GH, Back K (21 March 2022). "Functional Characterization of Serotonin N-Acetyltransferase in Archaeon Thermoplasma volcanium". Antioxidants. 11 (3): 596. doi:10.3390/antiox11030596. ISSN 2076-3921. PMC 8945778. PMID 35326246.
- Hoshino Y, Villanueva L (10 March 2023). "Four billion years of microbial terpenome evolution". FEMS Microbiology Reviews. 47 (2): fuad008. doi:10.1093/femsre/fuad008. ISSN 1574-6976. PMID 36941124.
- Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (October 2001). "Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus)". Journal of Agricultural and Food Chemistry. 49 (10): 4898–902. doi:10.1021/jf010321. PMID 11600041.
- González-Flores D, Velardo B, Garrido M, González-Gómez D, Lozano M, Ayuso MC, Barriga C, Paredes SD, Rodríguez AB (2011). "Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content". Journal of Food and Nutrition Research. 50 (4): 229–36.
- Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (May 2011). "Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection". Journal of Pineal Research. 50 (4): 374–80. doi:10.1111/j.1600-079X.2010.00853.x. PMID 21342247. S2CID 8034935.
- Salehi B (5 July 2019). "Melatonin in Medicinal and Food Plants" (PDF). Cells. 681. Archived from the original (PDF) on 29 November 2021. Retrieved 2 July 2021.
- Pereira N, Naufel MF, Ribeiro EB, Tufik S, Hachul H (January 2020). "Influence of Dietary Sources of Melatonin on Sleep Quality: A Review". Journal of Food Science. 85 (1). Wiley: 5–13. doi:10.1111/1750-3841.14952. PMID 31856339.
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, et al. (March 1995). "Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates". Biochemistry and Molecular Biology International. 35 (3): 627–34. PMID 7773197.
- Sae-Teaw M, Johns J, Johns NP, Subongkot S (August 2013). "Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers". Journal of Pineal Research. 55 (1): 58–64. doi:10.1111/jpi.12025. PMID 23137025. S2CID 979886.
External links
- "Melatonin". Chemwatch.
| Antioxidants | |
|---|---|
| Food antioxidants |
|
| Fuel antioxidants | |
| Measurements | |
| Tryptamines | |
|---|---|
| Tryptamines |
|
| N-Acetyltryptamines |
|
| α-Alkyltryptamines |
|
| Triptans | |
| Cyclized tryptamines |
|
| Isotryptamines | |
| Related compounds |
|