| Revision as of 12:52, 3 December 2016 edit47.201.179.7 (talk) Restore Ritchie333 See Discussion← Previous edit | Latest revision as of 02:30, 20 December 2024 edit undo2601:642:c303:f370:64e0:eb78:4b5b:1a7a (talk) ce | ||
| (653 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Vaccine to prevent poliomyelitis}} | |||
| {{Use dmy dates|date=November 2013}} | |||
| {{Good article}} | |||
| {{Drugbox | |||
| {{Use dmy dates|date=July 2024}} | |||
| | Verifiedfields = 449935826 | |||
| {{cs1 config |name-list-style=vanc |display-authors=6}} | |||
| | verifiedrevid = 450061491 | |||
| {{Infobox drug | |||
| | image = Poliodrops.jpg | |||
| | Verifiedfields = changed | |||
| <!-- Vacine data --> | |||
| | verifiedrevid = 450061491 | |||
| | type = vaccine | |||
| | image = Poliodrops.jpg | |||
| | target = ] | |||
| <!-- Vaccine data -->| type = vaccine | |||
| | vaccine_type = OPV: Attenuated; IPV: Killed | |||
| | target = ] | |||
| <!-- Clinical data --> | |||
| | vaccine_type = IPV: inactivated<br/>OPV: attenuated<br/>nOPV2: attenuated, genetically stabilised | |||
| | Drugs.com = {{drugs.com|MTM|polio_vaccine}} | |||
| <!-- Clinical data -->| tradename = Ipol, Poliovax, others | |||
| | pregnancy_AU = | |||
| | licence_CA = Imovax | |||
| | pregnancy_US = | |||
| | Drugs.com = {{drugs.com|monograph|poliovirus-vaccine-inactivated}} | |||
| | pregnancy_category = C (both OPV and IPV) | |||
| | MedlinePlus = a601177 | |||
| | legal_AU = | |||
| | |
| DailyMedID = Ipol | ||
| | pregnancy_AU = B2 | |||
| | legal_UK = | |||
| | pregnancy_AU_comment = <ref name="Drugs.com pregnancy">{{drugs.com|pregnancy|poliovirus-vaccine-inactivated}}</ref> | |||
| | legal_US = | |||
| | pregnancy_category = | |||
| | legal_status = Administered by or under the supervision of a health care professional. | |||
| | legal_AU = | |||
| | routes_of_administration = ] (IPV), By mouth (OPV) | |||
| | legal_CA = <!-- I, II, III, IV, V, VI, VII, VIII --> | |||
| <!-- Identifiers --> | |||
| | legal_UK = | |||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | legal_US = Rx-only | |||
| | ChemSpiderID = none | |||
| | legal_status = | |||
| | ATC_prefix = J07 | |||
| | routes_of_administration = IPV: ]<br/>OPV: ] | |||
| | ATC_suffix = BF01 | |||
| | ATC_prefix = J07 | |||
| | ATC_supplemental = {{ATC|J07|BF02}} {{ATC|J07|BF03}} | |||
| | ATC_suffix = BF01 | |||
| | PubChem = | |||
| | ATC_supplemental = {{ATC|J07|BF02}} {{ATC|J07|BF03}} {{ATC|J07|BF04}} | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| <!-- Identifiers -->| CAS_number = 1008119-78-5 | |||
| | DrugBank = | |||
| | PubChem = | |||
| <!--Chemical data --> | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB10796 | |||
| | DrugBank2 = DB10797 | |||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | ChemSpiderID = None | |||
| }} | }} | ||
| <!-- Definition and medical uses --> | <!-- Definition and medical uses --> | ||
| '''Polio vaccines''' are ]s used to prevent ] (polio).<ref name=WHO2014/> One type uses ] and is given by injection (IPV), while the other type uses ] and is given by mouth (OPV).<!-- <ref name=WHO2014/> --> The ] recommends all children be vaccinated against polio.<ref name=WHO2014/> The two vaccines have eliminated polio from most of the world,<ref name=Aylward_2006>{{cite journal |author=Aylward RB |title=Eradicating polio: today's challenges and tomorrow's legacy |journal=Annals of Tropical Medicine and Parasitology |volume=100 |issue=5–6 |pages=401–13 |year=2006 |pmid=16899145 |doi=10.1179/136485906X97354}}</ref><ref>{{cite journal |vauthors=Schonberger L, Kaplan J, Kim-Farley R, Moore M, Eddins D, Hatch M |title=Control of paralytic poliomyelitis in the United States |journal=Rev. Infect. Dis. |volume=6 Suppl 2 |pages=S424–6 |year=1984 |pmid=6740085 |doi=10.1093/clinids/6.Supplement_2.S424}}</ref> and reduced the number of cases each year from an estimated 350,000 in 1988 to 74 in 2015.<ref name=WHO2016Fs114>{{cite web|url = http://www.who.int/mediacentre/factsheets/fs114/en/|title = Poliomyelitis: Fact sheet N°114|publisher = World Health Organization|accessdate = 14 Sep 2016|date = Sep 2016}}</ref> | |||
| '''Polio vaccines''' are ]s used to prevent ] (polio).<ref name=WHO2016/><ref name=WHO2022>{{cite journal | vauthors = ((World Health Organization)) | title = Polio vaccines: WHO position paper – June 2022 | journal = Weekly Epidemiological Record | volume = 97 | issue = 25 | pages = 277–300 | date = 2022 | hdl=10665/357168 | hdl-access = free | author-link = World Health Organization }}</ref> Two types are used: an ] poliovirus given by injection (IPV) and a ] poliovirus given by mouth (OPV).<ref name=WHO2016/> The ] (WHO) recommends all children be fully vaccinated against polio.<ref name=WHO2016/> The two vaccines have eliminated polio from most of the world,<ref name="Aylward_2006">{{cite journal|vauthors=Aylward RB|year=2006|title=Eradicating polio: today's challenges and tomorrow's legacy|journal=Annals of Tropical Medicine and Parasitology|volume=100|issue=5–6|pages=401–413|doi=10.1179/136485906X97354|pmid=16899145|s2cid=25327986}}</ref><ref>{{cite journal|vauthors=Schonberger LB, Kaplan J, Kim-Farley R, Moore M, Eddins DL, Hatch M|year=1984|title=Control of paralytic poliomyelitis in the United States|journal=Reviews of Infectious Diseases|volume=6 | issue = Suppl 2|pages=S424–S426|doi=10.1093/clinids/6.Supplement_2.S424|pmid=6740085}}</ref> and reduced the number of cases reported each year from an estimated 350,000 in 1988 to 33 in 2018.<ref name="gwp2013">{{cite web|url=http://polioeradication.org/wp-content/uploads/2019/02/global-wild-poliovirus-2013-2018-20190201.pdf|title=Global Wild Poliovirus 2014–2019|access-date=3 February 2019|archive-date=3 February 2019|archive-url=https://web.archive.org/web/20190203085212/http://polioeradication.org/wp-content/uploads/2019/02/global-wild-poliovirus-2013-2018-20190201.pdf|url-status=live}}</ref><ref>{{Cite web|url=https://www.who.int/features/qa/07/en/|title=Does polio still exist? Is it curable?|website=] (WHO)|access-date=21 May 2018|archive-date=29 May 2018|archive-url=https://web.archive.org/web/20180529121019/http://www.who.int/features/qa/07/en/|url-status=live}}</ref> | |||
| <!-- Safety --> | <!-- Safety --> | ||
| The inactivated polio vaccines are very safe. |
The inactivated polio vaccines are very safe.<ref name=WHO2016/> Mild redness or pain may occur at the site of injection.<ref name=WHO2016/> Oral polio vaccines cause about three cases of vaccine-associated paralytic poliomyelitis per million doses given.<ref name=WHO2016/> This compares with 5,000 cases per million who are paralysed following a polio infection.<ref name="WHO2017Fs114">{{Cite web|url=https://www.who.int/mediacentre/factsheets/fs114/en/|title=Poliomyelitis|website=] (WHO)|archive-url=https://web.archive.org/web/20170418105535/http://www.who.int/mediacentre/factsheets/fs114/en/|archive-date=18 April 2017|url-status = live|access-date=25 April 2017}}</ref> Both types of vaccine are generally safe to give during ] and in those who have ] but are otherwise well.<ref name=WHO2016>{{cite journal | vauthors = ((World Health Organization)) | title = Polio vaccines: WHO position paper – March, 2016 | journal = Weekly Epidemiological Record | volume = 91 | issue = 12 | pages = 145–68 | date = 2016 | pmid = 27039410 | hdl=10665/254399 | hdl-access = free }}</ref> However, the emergence of circulating vaccine-derived poliovirus (cVDPV), a form of the vaccine virus that has reverted to causing poliomyelitis, has led to the development of novel oral polio vaccine type 2 (nOPV2) which aims to make the vaccine safer and thus stop further outbreaks of cVDPV.<ref name="GPEI-nOPV2">{{Cite web|title=GPEI-nOPV2|url=https://polioeradication.org/nopv2/|access-date=1 August 2021|language=en-GB|archive-date=27 July 2021|archive-url=https://web.archive.org/web/20210727211943/https://polioeradication.org/nopv2/|url-status=live}}</ref> | ||
| <!-- History, society and culture --> | <!-- History, society and culture --> | ||
| The first successful demonstration of a polio vaccine was by ] in 1950, with a live ] which people drank.<ref name="Kowproski_obit">{{cite web | vauthors = Fox M | url = https://www.nytimes.com/2013/04/21/us/hilary-koprowski-developed-live-virus-polio-vaccine-dies-at-96.html | title = Hilary Koprowski, Who Developed First Live-Virus Polio Vaccine, Dies at 96 | work = ] | date = 20 April 2013 | access-date = 8 September 2017 | archive-date = 25 August 2017 | archive-url = https://web.archive.org/web/20170825133630/http://www.nytimes.com/2013/04/21/us/hilary-koprowski-developed-live-virus-polio-vaccine-dies-at-96.html | url-status = live }}</ref> The vaccine was not approved for use in the United States, but was used successfully elsewhere.<ref name="Kowproski_obit"/> The success of an ] (killed) polio vaccine, developed by ], was ].<ref name=WHO2016/><ref>{{cite book| vauthors = Bazin H |title=Vaccination: A History|date=2011|publisher=John Libbey Eurotext|isbn=978-2742007752|page=395|url=https://books.google.com/books?id=orjaA_7sYZQC&pg=PA395|url-status = live|archive-url=https://web.archive.org/web/20170908183608/https://books.google.com/books?id=orjaA_7sYZQC&pg=PA395|archive-date=8 September 2017}}</ref> Another attenuated live oral polio vaccine was developed by ] and came into commercial use in 1961.<ref name=WHO2016/><ref>{{cite journal|vauthors=Smith DR, Leggat PA|date=2005|title=Pioneering figures in medicine: Albert Bruce Sabin – inventor of the oral polio vaccine|journal=The Kurume Medical Journal|volume=52|issue=3|pages=111–116|doi=10.2739/kurumemedj.52.111|pmid=16422178|doi-access=free}}</ref> | |||
| The first polio vaccine was the inactivated polio vaccine.<!-- <ref name=WHO2014/> --> It was developed by ] and came into use in 1955.<ref name=WHO2014/> The Pasteur Institute in Paris nearly simultaneously announced an effective polio vaccine<ref>The Pasteur Institute stated that an anti-poliomyelitis vaccine, developed by Professor Pierre Lepine would soon be produced in large quantities. (Times, London, April 4, 1955).</ref> <ref>http://webext.pasteur.fr/archives/e_lep0.html</ref> <ref>https://books.google.com/books?id=iKidtL80imMC&pg=PA90&lpg=PA90&dq=pierre+lepine+polio&source=bl&ots=yLfOrmqZQ-&sig=Jb8FAVuKoWoFVmspQroxxVJPiSQ&hl=en&sa=X&ved=0ahUKEwjX6ayShszQAhUJbSYKHVGjA_oQ6AEIUDAL#v=onepage&q=pierre%20lepine%20polio&f=false</ref> The oral polio vaccine was developed by ] and came into commercial use in 1961.<ref name=WHO2014/><ref>{{cite journal|last1=Smith|first1=DR|last2=Leggat|first2=PA|title=Pioneering figures in medicine: Albert Bruce Sabin--inventor of the oral polio vaccine.|journal=The Kurume medical journal|date=2005|volume=52|issue=3|pages=111–6|pmid=16422178|doi=10.2739/kurumemedj.52.111}}</ref> They are on the ], the most important medication needed in a basic ].<ref>{{cite web|title=WHO Model List of EssentialMedicines|url=http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1|work=World Health Organization|accessdate=22 April 2014|date=October 2013}}</ref> The wholesale cost in the ] is about 0.25 USD per dose for the oral form as of 2014.<ref name="Vaccine, Polio">{{cite web|title=Vaccine, Polio|url=http://erc.msh.org/dmpguide/resultsdetail.cfm?language=english&code=POL00X&s_year=2014&year=2014&str=&desc=Vaccine%2C%20Polio&pack=new&frm=VIAL&rte=PO&class_code2=19%2E3%2E&supplement=&class_name=%2819%2E3%2E%29Vaccines%3Cbr%3E|website=International Drug Price Indicator Guide|accessdate=6 December 2015}}</ref> In the United States it costs between 25 and 50 USD for the inactivated form.<ref name=Ric2015>{{cite book|last1=Hamilton|first1=Richart|title=Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition|date=2015|publisher=Jones & Bartlett Learning|isbn=978-1-284-05756-0|page=316}}</ref> | |||
| Polio vaccine is on the ].<ref name="WHO23rd">{{cite book | vauthors = ((World Health Organization)) | title = The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023) | year = 2023 | hdl = 10665/371090 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MHP/HPS/EML/2023.02 | hdl-access=free }}</ref> | |||
| {{TOC limit|3}} | |||
| ==Medical uses== | ==Medical uses== | ||
| ], the "Wellbee", encouraging the public to receive an oral polio vaccine.]] | ]'s national symbol of ], the "]", encouraging the public to receive an oral polio vaccine.]] | ||
| Interruption of person-to-person transmission of the virus by vaccination is important in |
Interruption of person-to-person transmission of the virus by vaccination is important in global ],<ref name="Fine">{{cite journal|vauthors=Fine PE, Carneiro IA|date=November 1999|title=Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative|journal=American Journal of Epidemiology|volume=150|issue=10|pages=1001–1021|doi=10.1093/oxfordjournals.aje.a009924|pmid=10568615|doi-access=free}}</ref> since no long-term ] state exists for ] in individuals with normal immune function, polio viruses have no non-primate reservoir in nature,<ref>{{cite journal|vauthors=Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, Nomoto A|date=February 1991|title=Transgenic mice susceptible to poliovirus|journal=Proc. Natl. Acad. Sci. U.S.A.|volume=88|issue=3|pages=951–955|bibcode=1991PNAS...88..951K|doi=10.1073/pnas.88.3.951|pmc=50932|pmid=1846972|doi-access=free}}</ref> and survival of the virus in the environment for an extended period appears to be remote. There are two types of vaccine: inactivated polio vaccine (IPV) and oral polio vaccine (OPV). | ||
| ===Inactivated=== | ===Inactivated=== | ||
| When the |
When the IPV (injection) is used, 90% or more of individuals develop protective antibodies to all three ]s of ] after two doses of inactivated polio vaccine (IPV), and at least 99% are immune to poliovirus following three doses. The duration of immunity induced by IPV is not known with certainty, although a complete series is thought to protect for many years.<ref name=Robertson_1993>{{cite book | vauthors = Robertson S | title = Module 6: Poliomyelitis | series = The Immunological Basis for Immunization Series | publisher = ] (WHO) | url = https://www.who.int/ihr/polio1993en.pdf | access-date = 18 October 2019 |archive-url=https://web.archive.org/web/20191019064736/https://www.who.int/ihr/polio1993en.pdf |archive-date=19 October 2019 | id = WHO/EPI/GEN/93.16 }}</ref> IPV replaced the oral vaccine in many developed countries in the 1990s mainly due to the (small) risk of vaccine-derived polio in the oral vaccine.<ref>{{Cite web|author=Public Health Agency of Canada|date=18 July 2007|title=Poliomyelitis vaccine: Canadian Immunization Guide|url=https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-17-poliomyelitis-vaccine.html|access-date=1 August 2021|website=www.canada.ca|archive-date=27 July 2021|archive-url=https://web.archive.org/web/20210727212739/https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-17-poliomyelitis-vaccine.html|url-status=live}}</ref><ref>{{Cite web|date=25 March 2021|title=Polio Vaccination: What Everyone Should Know {{!}} CDC|url=https://www.cdc.gov/vaccines/vpd/polio/public/index.html|access-date=1 August 2021|website=www.cdc.gov|language=en-us|archive-date=16 January 2021|archive-url=https://web.archive.org/web/20210116130844/https://www.cdc.gov/vaccines/vpd/polio/public/index.html|url-status=live}}</ref> | ||
| ===Attenuated=== |
===Attenuated=== | ||
| Oral polio vaccines were easier to administer than IPV, as they eliminated the need for sterile syringes and therefore were more suitable for mass vaccination campaigns. OPV also provided longer-lasting ] than the Salk vaccine, as it provides both ] and ].<ref name="ReferenceZ">{{cite journal|vauthors=Wahid R, Cannon MJ, Chow M|date=May 2005|title=Virus-Specific CD4+ and CD8+ Cytotoxic T-Cell Responses and Long-Term T-Cell Memory in Individuals Vaccinated against Polio|journal=Journal of Virology|volume=79|issue=10|pages=5988–5995|doi=10.1128/JVI.79.10.5988-5995.2005|pmc=1091702|pmid=15857985}}</ref> | |||
| One dose of trivalent OPV produces immunity to all three poliovirus serotypes in roughly 50% of recipients.<ref name=PinkPages/> Three doses of live-attenuated OPV produce protective antibodies to all three poliovirus types in more than 95% of recipients. As with other live-virus vaccines, immunity initiated by OPV is probably lifelong.<ref name="Robertson_1993" /> OPV produces excellent immunity in the ], the primary site of wild poliovirus entry, which helps prevent infection with wild virus in areas where the virus is ].<ref name=Peds/> The oral administration does not require special medical equipment or extensive training. Attenuated poliovirus derived from the oral polio vaccine is excreted for a few days after vaccination, potentially infecting and thus indirectly inducing ] in unvaccinated individuals, and thus amplifying the effects of the doses delivered.<ref name="Nathan">{{cite journal |vauthors=Nathanson N, Martin JR |date=December 1979 |title=The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance |journal=American Journal of Epidemiology |volume=110 |issue=6 |pages=672–692 |doi=10.1093/oxfordjournals.aje.a112848 |pmid=400274}}</ref> Taken together, these advantages have made it the favored vaccine of many countries, and it has long been preferred by the global eradication initiative.<ref name="GPEI-OPV">{{cite web |title=OPV - Oral Polio Vaccine |url=https://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/ |archive-url= |archive-date= |access-date=9 August 2024 |website=Global Polio Eradication Initiative (GPEI) |publisher=}}</ref>]The primary disadvantage of OPV derives from its inherent nature. As an attenuated but active virus, it can induce vaccine-associated paralytic poliomyelitis (VAPP) in approximately one individual per every 2.7{{nbsp}}million doses administered.<ref name="GPEI-OPV" /> The live virus can circulate in under-vaccinated populations (termed either '''variant poliovirus''' or '''circulating vaccine-derived poliovirus''', cVDPV) and over time can revert to a neurovirulent form causing paralytic polio.<ref name="GPEI-OPV" /> This genetic reversal of the pathogen to a virulent form takes a considerable time and does not affect the person who was originally vaccinated. With wild polio cases at record lows, 2017 was the first year where more cases of cVDPV were recorded than the wild poliovirus.<ref>{{Cite web |title=Eradication of polio – Is Syria being left behind? |url=https://sphcm.med.unsw.edu.au/infectious-diseases-blog/eradication-polio-syria-being-left-behind |url-status=live |archive-url=https://web.archive.org/web/20181006114450/https://sphcm.med.unsw.edu.au/infectious-diseases-blog/eradication-polio-syria-being-left-behindd |archive-date=6 October 2018 |access-date=6 October 2018 |website=UNSW School of Public Health and Community Medicine |vauthors=Kunasekaran M}}</ref> | |||
| Until recent times, a trivalent OPV containing all three virus strains was used, and had nearly eradicated polio infection worldwide.<ref name="ReferenceA">{{cite journal |vauthors=Marin M, Patel M, Oberste S, Pallansch MA |date=January 2017 |title=Guidance for Assessment of Poliovirus Vaccination Status and Vaccination of Children Who Have Received Poliovirus Vaccine Outside the United States |journal=MMWR. Morbidity and Mortality Weekly Report |volume=66 |issue=1 |pages=23–25 |doi=10.15585/mmwr.mm6601a6 |pmc=5687270 |pmid=28081056}}</ref> With the complete eradication of wild poliovirus type{{nbsp}}2<ref>{{cite web |date=22 July 2019 |title=Poliomyelitis |url=https://www.who.int/news-room/fact-sheets/detail/poliomyelitis |url-status=live |archive-url=https://web.archive.org/web/20191017233318/https://www.who.int/news-room/fact-sheets/detail/poliomyelitis |archive-date=17 October 2019 |access-date=18 October 2019 |website=] (WHO)}}</ref> this was phased out in 2016 and replaced with bivalent vaccine containing just types 1 and 3, supplemented with monovalent type{{nbsp}}2 OPV in regions where cVDPV type 2 was known to circulate.<ref name="GPEI-OPV" /> The switch to the bivalent vaccine and associated missing immunity against type 2 strains, among other factors, led to outbreaks of circulating vaccine-derived poliovirus type 2 (cVDPV2), which increased from 2 cases in 2016 to 1037 cases in 2020.<ref>{{Cite web |title=GPEI Strategy for the Response to cVDPV2 2020–2021 |url=https://polioeradication.org/wp-content/uploads/2021/03/GPEI-cVDPV2-nOPV2-Factsheet-20210312-EN.pdf |url-status=live |archive-url=https://web.archive.org/web/20210801223541/https://polioeradication.org/wp-content/uploads/2021/03/GPEI-cVDPV2-nOPV2-Factsheet-20210312-EN.pdf |archive-date=1 August 2021 |access-date=1 August 2021 |website=Polio Global Eradication Initiative}}</ref> | |||
| One dose of OPV produces immunity to all three poliovirus serotypes in approximately 50% of recipients.<ref name=PinkPages/> Three doses of live-attenuated OPV produce protective antibodies to all three poliovirus types in more than 95% of recipients. OPV produces excellent immunity in the ], the primary site of wild poliovirus entry, which helps prevent infection with wild virus in areas where the virus is ].<ref name=Peds/> The live virus used in the vaccine is shed in the stool and can be spread to others within a community. The live virus also has stringent requirements for transport and storage, which are a problem in some hot or remote areas. As with other live-virus vaccines, immunity initiated by OPV is probably lifelong.<ref name=Robertson_1993/> | |||
| A novel OPV2 vaccine (nOPV2) which has been genetically modified to reduce the likelihood of disease-causing activating mutations was granted emergency licencing in 2021, and subsequently full licensure in December 2023.<ref name=":x22">{{Cite web |date=12 April 2024 |title=GPEI-OPV Oral polio vaccine |url=https://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/ |access-date=12 April 2024 |website=Global Polio Eradication Initiative - World Health Organization |language=en-GB}}</ref> This has greater genetic stability than the traditional oral vaccine and is less likely to revert to a virulent form.<ref name=":x2">{{Cite web |date=12 April 2024 |title=GPEI-OPV Oral polio vaccine |url=https://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/ |access-date=12 April 2024 |website=Global Polio Eradication Initiative - World Health Organization |language=en-GB}}</ref><ref name="GPEI-nOPV2" /> Genetically stabilised vaccines targeting poliovirus types 1 and 3 are in development, with the intention that these will eventually completely replace the Sabin vaccines.<ref>{{Cite web |last=Gadye |first=Levi |date=2023-06-14 |title=Two New Vaccines Join the Fight to Eradicate Polio {{!}} UC San Francisco |url=https://www.ucsf.edu/news/2023/06/425601/two-new-vaccines-join-fight-eradicate-polio |access-date=2024-08-10 |website=The University of California San Francisco |language=en}}</ref> | |||
| The trivalent (against ] 1, 2 and 3) OPV has been used and nearly eradicated polio infection worldwide. Spearheaded by The Global Polio Eradication Initiative, 155 countries switched to use the bivalent (against wild type 1 and 3) between 17 April and 1 May 2016.<ref>{{cite web|url=http://www.fiercepharma.com/vaccines/worldwide-switch-to-bivalent-oral-polio-vaccine-to-kick-off-on-Sunday|title=Worldwide switch to bivalent oral polio vaccine to kick off on Sunday|author=Al Idrus, Amirah|publisher=fiercepharma.com|date=13 April 2016|accessdate=1 May 2016}}</ref> The bivalent OPV is at least 30% more effective than the trivalent one.<ref>{{cite web|url=http://www.polioeradication.org/Polioandprevention/Thevaccines/BivalentOPV.aspx|title=Bivalent oral polio vaccine|author=Polio Eradication Initiative|publisher=Polio Eradication Initiative|accessdate=1 May 2016}}</ref> | |||
| ===Schedule=== | ===Schedule=== | ||
| ] | |||
| The World Health Organization recommends three or four doses starting at two months of age.<ref name=WHO2014/> It can be begun earlier but then additional doses are needed.<ref name=WHO2014/> | |||
| In countries with endemic polio or where the risk of imported cases is high, the WHO recommends OPV vaccine at birth followed by a primary series of three OPV doses and at least one IPV dose starting at 6 weeks of age, with a minimum of 4 weeks between OPV doses. In countries with >90% immunization coverage and low risk of importation, the WHO recommends one or two IPV doses starting at 2 months of age followed by at least two OPV doses, with the doses separated by 4–8 weeks depending on the risk of exposure. In countries with the highest levels of coverage and the lowest risks of importation and transmission, the WHO recommends a primary series of three IPV injections, with a booster dose after an interval of six months or more if the first dose was administered before 2 months of age.<ref name="WHO2016" /> | |||
| ==Side effects== | ==Side effects== | ||
| The inactivated polio vaccines are very safe. Mild redness or pain may occur at the site of injection. They are generally safe to be given to ] women and those who have ] but are otherwise well.<ref name="WHO2016" /> | |||
| ] for use in a 1967 vaccination campaign in ], ]]] | |||
| The inactivated polio vaccines are very safe.<!-- <ref name=WHO2014/> --> Mild redness or pain may occur at the site of injection.<!-- <ref name=WHO2014/> --> Oral polio vaccine results in vaccine-associated paralytic poliomyelitis in about three per million doses.<!-- <ref name=WHO2014/> --> They are generally safe to give during ] and in those who have ] but are otherwise well.<ref name=WHO2014/> | |||
| === Allergic reaction to the vaccine === | |||
| Inactivated polio vaccine can cause an allergic reaction in a few people since the vaccine contains trace amounts of ], ], ], and ]. It should not be given to anyone who has an allergic reaction to these medicines. Signs and symptoms of an allergic reaction, which usually appear within minutes or a few hours after receiving the injected vaccine, include breathing difficulties, weakness, hoarseness or wheezing, heart rate fluctuations, skin rash, and dizziness.<ref>{{Cite web|title=Common and Rare Side Effects for poliovirus vaccine injection|url=https://www.webmd.com/drugs/2/drug-14411/poliovirus-vaccine-injection/details/list-sideeffects|access-date=24 August 2021|website=www.webmd.com|archive-date=24 August 2021|archive-url=https://web.archive.org/web/20210824070346/https://www.webmd.com/drugs/2/drug-14411/poliovirus-vaccine-injection/details/list-sideeffects|url-status=live}}</ref> | |||
| ===Vaccine- |
=== Vaccine-associated paralytic polio === | ||
| A potential adverse effect of the Sabin OPV is caused by its known potential to recombine to a form that causes neurological infection and paralysis.<ref name="Shimizu_2004">{{cite journal | vauthors = Shimizu H, Thorley B, Paladin FJ, Brussen KA, Stambos V, Yuen L, Utama A, Tano Y, Arita M, Yoshida H, Yoneyama T, Benegas A, Roesel S, Pallansch M, Kew O, Miyamura T | title = Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001 | journal = Journal of Virology | volume = 78 | issue = 24 | pages = 13512–13521 | date = December 2004 | pmid = 15564462 | pmc = 533948 | doi = 10.1128/JVI.78.24.13512-13521.2004 }}</ref> The Sabin OPV results in vaccine-associated paralytic poliomyelitis (VAPP) in approximately one individual per every 2.7{{nbsp}}million doses administered, with symptoms identical to wild polio.<ref name="GPEI-OPV" /> Due to its improved genetic stability, the novel OPV (nOPV) has a reduced risk of this occurring.<ref>{{Cite web |date=April 2023 |title=nOPV2: Clinical Development and Evidence Summary |url=https://polioeradication.org/wp-content/uploads/2023/05/EN_nOPV2-Clinical-Development-Summary_Apr-2023.pdf |access-date=10 August 2024 |website=Global Polio Eradication Initiative}}</ref> | |||
| A potential, but rare, adverse effect of the oral polio vaccine (OPV) is its known ability to recombine to a form that may cause neurological infection and cause paralysis.<ref name=Shimizu_2004>{{cite journal |author=Shimizu H |title=Circulation of Type 1 Vaccine-Derived Poliovirus in the Philippines in 2001 |journal=J. Virol. |volume=78 |issue=24 |pages=13512–21 |date=December 2004 |pmid=15564462 |pmc=533948 |doi=10.1128/JVI.78.24.13512-13521.2004 |url=http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=15564462 |name-list-format=vanc|author2=Thorley B |author3=Paladin FJ |display-authors=3 |last4=Brussen |first4=K. A. |last5=Stambos |first5=V. |last6=Yuen |first6=L. |last7=Utama |first7=A. |last8=Tano |first8=Y. |last9=Arita |first9=M.}}</ref> Clinical disease, including paralysis, caused by vaccine-derived poliovirus (VDPV) is indistinguishable from that caused by wild polioviruses.<ref name=Cono>{{cite web|vauthors=Cono J, Alexander LN | year= 2002 | work=Vaccine-Preventable Disease Surveillance Manual | edition=3rd | title=Chapter 10: Poliomyelitis | url= http://cdc.gov/vaccines//pubs/surv-manual/3rd-edition-chpt10_polio.pdf | archiveurl= https://web.archive.org/web/20111022064610/http://cdc.gov/vaccines//pubs/surv-manual/3rd-edition-chpt10_polio.pdf | archivedate= 2011-10-22|format=PDF}}</ref> This is believed to be a rare event, but outbreaks of vaccine-associated paralytic poliomyelitis (VAPP), caused by a circulating vaccine-derived poliovirus (cVDPV),<ref name="who-Q&A">{{cite web | title = What is vaccine-derived polio? | website = Online Q&A - WHO | date = October 2014| url = http://www.who.int/features/qa/64/en/ | accessdate = 2015-09-06}}</ref> have been reported, and tend to occur in areas of low coverage by OPV, presumably because the OPV is itself protective against the related outbreak strain.<ref name=Kew_2002>{{cite journal | author = Kew O | title = Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus | journal = Science | volume = 296 | issue = 5566 | pages = 356–9 | year = 2002 | pmid = 11896235 | doi = 10.1126/science.1068284 |name-list-format=vanc | display-authors = 1 | last2 = Morris-Glasgow | first2 = V | last3 = Landaverde | first3 = M | last4 = Burns | first4 = C | last5 = Shaw | first5 = J | last6 = Garib | first6 = Z | last7 = André | first7 = J | last8 = Blackman | first8 = E | last9 = Freeman | first9 = CJ}}</ref><ref name=Yang_2003>{{cite journal |author=Yang CF |title=Circulation of Endemic Type 2 Vaccine-Derived Poliovirus in Egypt from 1983 to 1993 |journal=J. Virol. |volume=77 |issue=15 |pages=8366–77 |date=August 2003 |pmid=12857906 |pmc=165252 |doi= 10.1128/JVI.77.15.8366-8377.2003|url=http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=12857906 |name-list-format=vanc|author2=Naguib T |author3=Yang SJ |display-authors=3 |last4=Nasr |first4=E. |last5=Jorba |first5=J. |last6=Ahmed |first6=N. |last7=Campagnoli |first7=R. |last8=Van Der Avoort |first8=H. |last9=Shimizu |first9=H.}}</ref> | |||
| ===Contamination concerns=== | ===Contamination concerns=== | ||
| {{further|Vaccine contamination with SV40}} | |||
| In 1960, it was determined that the ] kidney cells used to prepare the poliovirus vaccines were infected with the ] virus (Simian Virus-40).<ref name=SV>{{cite web| title = Simian Virus 40 (SV40), Polio Vaccine, and Cancer | work = Vaccine Safety | publisher = Centers for Disease Control| date = 2004-04-22 | url = http://www.cdc.gov/vaccinesafety/updates/archive/polio_and_cancer_factsheet.htm | archiveurl = https://web.archive.org/web/20130522091608/http://www.cdc.gov/vaccinesafety/updates/archive/polio_and_cancer_factsheet.htm | archivedate = 2013-05-22 | accessdate = 2013-05-22}}</ref> SV40 was also discovered in 1960 and is a naturally occurring ] that infects monkeys. In 1961, SV40 was found to cause tumors in ]s.<ref name=Eddy_1961>{{cite journal |vauthors=Eddy B, Borman G, Berkeley W, Young R |title=Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts |journal=Proc Soc Exp Biol Med |volume=107 |pages=191–7 |year = 1961 |pmid = 13725644 |doi=10.3181/00379727-107-26576}}</ref> More recently, the virus was found in certain forms of ] in humans, for instance ] and ]s, ] and ] mesothelioma, and some types of ].<ref>{{cite journal |author=Carbone M |title=Simian virus 40 and human tumors: It is time to study mechanisms |journal=J Cell Biochem |volume=76 |issue=2 |pages=189–93 |year=1999 |pmid = 10618636 |doi=10.1002/(SICI)1097-4644(20000201)76:2<189::AID-JCB3>3.0.CO;2-J}}</ref><ref>{{cite journal |vauthors=Vilchez R, Kozinetz C, Arrington A, Madden C, Butel J |title=Simian virus 40 in human cancers |journal=Am J Med |volume=114 |issue=8 |pages=675–84 |year=2003 |pmid=12798456 |doi=10.1016/S0002-9343(03)00087-1}}</ref> However, it has not been determined that SV40 causes these cancers.<ref name=Engels>{{cite journal |author=Engels E |title=Cancer risk associated with receipt of vaccines contaminated with simian virus 40: epidemiologic research |url=http://dceg.cancer.gov/pdfs/engels41972005.pdf |format=PDF|journal=Expert Rev Vaccines |volume=4 |issue=2 |pages=197–206 |year=2005 |pmid=15889993 |doi=10.1586/14760584.4.2.197 |archiveurl=https://web.archive.org/web/20060923085542/http://dceg.cancer.gov/pdfs/engels41972005.pdf |archivedate=2006-09-23}}</ref> | |||
| In 1960, the ] kidney cells used to prepare the poliovirus vaccines were determined to be infected with the ] (SV40),<ref name=SV>{{cite web| title = Simian Virus 40 (SV40), Polio Vaccine, and Cancer | work = Vaccine Safety | publisher = Centers for Disease Control| date = 22 April 2004 | url = https://www.cdc.gov/vaccinesafety/updates/archive/polio_and_cancer_factsheet.htm | archive-url = https://web.archive.org/web/20130522091608/http://www.cdc.gov/vaccinesafety/updates/archive/polio_and_cancer_factsheet.htm | archive-date = 22 May 2013 | access-date = 22 May 2013}}</ref> which was also discovered in 1960 and is a naturally occurring ] that infects monkeys. In 1961, SV40 was found to cause tumors in ]s.<ref name="Eddy_1961">{{cite journal|vauthors=Eddy BE, Borman GS, Berkeley WH, Young RD|date=May 1961|title=Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts|journal=Proceedings of the Society for Experimental Biology and Medicine|volume=107|pages=191–197|doi=10.3181/00379727-107-26576|pmid=13725644|s2cid=31275908}}</ref> More recently, the virus was found in certain forms of ] in humans, for instance ] and ]s, ] and ] mesothelioma, and some types of ].<ref>{{cite journal|vauthors=Carbone M|date=December 1999|title=Simian virus 40 and human tumors: It is time to study mechanisms|journal=Journal of Cellular Biochemistry|volume=76|issue=2|pages=189–193|doi=10.1002/(SICI)1097-4644(20000201)76:2<189::AID-JCB3>3.0.CO;2-J|pmid=10618636|s2cid=795975 }}</ref><ref>{{cite journal|vauthors=Vilchez RA, Kozinetz CA, Arrington AS, Madden CR, Butel JS|date=June 2003|title=Simian virus 40 in human cancers|journal=The American Journal of Medicine|volume=114|issue=8|pages=675–684|doi=10.1016/S0002-9343(03)00087-1|pmid=12798456}}</ref> However, SV40 has not been determined to cause these cancers.<ref name=Engels>{{cite journal | vauthors = Engels EA | s2cid = 5861910 | title = Cancer risk associated with receipt of vaccines contaminated with simian virus 40: epidemiologic research | journal = Expert Review of Vaccines | volume = 4 | issue = 2 | pages = 197–206 | date = April 2005 | pmid = 15889993 | doi = 10.1586/14760584.4.2.197 | url = https://zenodo.org/record/1235758 | access-date = 30 June 2019 | archive-date = 20 April 2020 | archive-url = https://web.archive.org/web/20200420144252/https://zenodo.org/record/1235758 | url-status = live }}</ref> | |||
| SV40 was found to be present in stocks of the injected form of the |
SV40 was found to be present in stocks of the injected form of the IPV in use between 1955 and 1963.<ref name=SV/> It is not found in the OPV form.<ref name=SV/> Over 98 million Americans received one or more doses of polio vaccine between 1955 and 1963 when a proportion of vaccine was contaminated with SV40; an estimated 10–30 million Americans may have received a dose of vaccine contaminated with SV40.<ref name=SV/> Later analysis suggested that vaccines produced by the former ] countries until 1980, and used in the ], ], ], and several ]n countries, may have been contaminated, meaning hundreds of millions more may have been exposed to SV40.<ref>{{cite magazine | vauthors = Bookchin D| title = Vaccine scandal revives cancer fear | magazine = New Scientist | date = 7 July 2004 |url=https://www.newscientist.com/news/news.jsp?id=ns99996116| archive-url = https://web.archive.org/web/20040720074452/http://www.newscientist.com/news/news.jsp?id=ns99996116| archive-date=20 July 2004 | access-date = 29 November 2008 }}</ref> | ||
| In 1998, the ] undertook a large study, using cancer case information from the |
In 1998, the ] undertook a large study, using cancer case information from the institute's SEER database. The published findings from the study revealed no increased incidence of cancer in persons who may have received vaccine containing SV40.<ref name="Strickler_1998">{{cite journal|vauthors=Strickler HD, Rosenberg PS, Devesa SS, Hertel J, Fraumeni JF, Goedert JJ|date=January 1998|title=Contamination of poliovirus vaccines with simian virus 40 (1955–1963) and subsequent cancer rates|journal=JAMA|volume=279|issue=4|pages=292–295|doi=10.1001/jama.279.4.292|pmid=9450713|doi-access=free}}</ref> Another large study in Sweden examined cancer rates of 700,000 individuals who had received potentially contaminated polio vaccine as late as 1957; the study again revealed no increased cancer incidence between persons who received polio vaccines containing SV40 and those who did not.<ref name="Olin_1998">{{cite journal|vauthors=Olin P, Giesecke J|year=1998|title=Potential exposure to SV40 in polio vaccines used in Sweden during 1957: no impact on cancer incidence rates 1960 to 1993|journal=Developments in Biological Standardization|volume=94|pages=227–233|pmid=9776244}}</ref> The question of whether SV40 causes cancer in humans remains controversial, however, and the development of improved assays for detection of SV40 in human tissues will be needed to resolve the controversy.<ref name=Engels/> | ||
| ]s for use in a 1967 vaccination campaign in ], ]]] | |||
| During the race to develop an oral polio vaccine several large |
During the race to develop an oral polio vaccine, several large-scale human trials were undertaken. By 1958, the National Institutes of Health had determined that OPV produced using the Sabin strains was the safest.<ref name=Sanofi/> Between 1957 and 1960, however, ] continued to administer his vaccine around the world. In Africa, the vaccines were administered to roughly one million people in the Belgian territories (now the ], ], and ]).<ref name="Plotkin">{{cite journal|vauthors=Plotkin SA|date=April 2001|title=CHAT oral polio vaccine was not the source of human immunodeficiency virus type 1 group M for humans|journal=Clinical Infectious Diseases|volume=32|issue=7|pages=1068–1084|doi=10.1086/319612|pmid=11264036|doi-access=free}}</ref><ref name=Kowproski5>{{cite journal | vauthors = Koprowski H | title = Historical aspects of the development of live virus vaccine in poliomyelitis | journal = British Medical Journal | volume = 2 | issue = 5192 | pages = 85–91 | date = July 1960 | pmid = 14410975 | pmc = 2096806 | doi = 10.1136/bmj.2.5192.85 }}</ref> The results of these human trials have been controversial,<ref name=Collins_2000>{{cite news | vauthors = Collins H | title = The Gulp Heard Round the World | page = D-1 | website = The Philadelphia Inquirer | date = 6 November 2000 | url = http://www.koprowski.net/Polio+Article.htm | access-date = 29 November 2008 |url-status = dead| archive-url = https://web.archive.org/web/20040405233046/http://www.koprowski.net/Polio%20Article.htm | archive-date = 5 April 2004 | df = dmy-all }}</ref> and unfounded ] arose that the vaccine had created the conditions necessary for transmission of ] from ]s to humans, causing ]. These hypotheses, however, ].<ref name="Plotkin" /> By 2004, cases of poliomyelitis in Africa had been reduced to just a small number of isolated regions in the western portion of the continent, with sporadic cases elsewhere. Recent local opposition to vaccination campaigns have evolved due to lack of adequate information,<ref>{{cite news |url=http://news.bbc.co.uk/2/hi/africa/2070634.stm |publisher=BBC News Online |title=Nigeria Muslims oppose polio vaccination |date=27 June 2002 |access-date=29 November 2008 |url-status = live|archive-url=https://web.archive.org/web/20081129152933/http://news.bbc.co.uk/2/hi/africa/2070634.stm |archive-date=29 November 2008 }}</ref><ref>{{cite news |vauthors=Dugger CW, McNeil DG |url=https://www.nytimes.com/2006/03/20/international/asia/20polio.html |title=Rumor, Fear and Fatigue Hinder Final Push to End Polio |work=] |date=20 March 2006 |access-date=29 November 2008 |url-status = live|archive-url=https://web.archive.org/web/20081210212451/http://www.nytimes.com/2006/03/20/international/asia/20polio.html?pagewanted=2 |archive-date=10 December 2008 }}</ref> often relating to fears that the vaccine might induce ].<ref>{{cite news |url=http://news.bbc.co.uk/2/hi/africa/4539757.stm |publisher=] |title=Anti-polio vaccine Malians jailed |date=12 May 2005 |access-date=29 November 2008 |url-status = live|archive-url=https://web.archive.org/web/20060110000559/http://news.bbc.co.uk/2/hi/africa/4539757.stm |archive-date=10 January 2006 }}</ref> The disease has since resurged in ] and in several other African nations without necessary information, which ]s believe is due to refusals by certain local populations to allow their children to receive the polio vaccine.<ref name="Jegede"/> | ||
| ==Manufacture== | ==Manufacture== | ||
| ===Inactivated=== | ===Inactivated=== | ||
| The Salk vaccine, |
The Salk vaccine, IPV, is based on three wild, ] reference strains, Mahoney (type 1 poliovirus), MEF-1 (type 2 poliovirus), and Saukett (type 3 poliovirus), grown in a type of monkey ] tissue culture (] line), which are then inactivated with ].<ref name=Kew_2005/> The injected Salk vaccine confers ]-mediated immunity in the bloodstream, which prevents polio infection from progressing to ] and protects the ], thus eliminating the risk of ] and ]. | ||
| In the United States, vaccine is administered along with the ], ], and ] ] vaccines (]) and a pediatric dose of ] vaccine.<ref name = PinkPages/> In the UK, IPV is combined with tetanus, diphtheria, pertussis, and '']'' type b vaccines.<ref name=UK>{{cite book | |
In the United States, the vaccine is administered along with the ], ], and ] ] vaccines (]) and a pediatric dose of ] vaccine.<ref name = PinkPages/> In the UK, IPV is combined with tetanus, diphtheria, pertussis, and '']'' type b vaccines.<ref name="UK">{{cite book|title=Immunisation Against Infectious Disease|vauthors=((Joint Committee on Vaccination and Immunisation))|publisher=Stationery Office|year=2006|isbn=978-0-11-322528-6|veditors=Salisbury D, Ramsay M, Noakes K|location=Edinburgh|pages=313–329|chapter=26: Poliomyelitis|chapter-url=http://www.immunisation.nhs.uk/files/GB_26_polio.pdf|archive-url=https://web.archive.org/web/20070615150746/http://www.immunisation.nhs.uk/files/GB_26_polio.pdf|archive-date=15 June 2007}}</ref> | ||
| ===Attenuated=== | ===Attenuated=== | ||
| ] | |||
| Oral polio vaccine (OPV) is an ], produced by the passage of the virus through non-human cells at a sub-] temperature, which produces spontaneous mutations in the viral genome.<ref>{{cite journal |author=Sabin A |title=Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses |journal=JAMA |volume=173 |pages=1521–6 |year= 1960 |pmid = 14440553 |name-list-format=vanc|author2=Ramos-Alvarez M |author3=Alvarez-Amezquita J |display-authors=3 |last4=Pelon |first4=W |last5=Michaels |first5=RH |last6=Spigland |first6=I |last7=Koch |first7=MA |last8=Barnes |first8=JM |last9=Rhim |first9=JS |doi=10.1001/jama.1960.03020320001001 |issue=14}}</ref> Oral polio vaccines were developed by several groups, one of which was led by ]. Other groups, led by ] and ], developed their own attenuated vaccine strains. In 1958, the ] created a special committee on live polio vaccines. The various vaccines were carefully evaluated for their ability to induce immunity to polio, while retaining a low incidence of neuropathogenicity in monkeys. Large-scale clinical trials performed in the Soviet Union in late 1950s to early 1960s by ] and his colleagues demonstrated safety and high efficacy of the vaccine.<ref>{{cite journal |author=Sabin AB |title=Role of my cooperation with Soviet scientists in the elimination of polio: possible lessons for relations between the U.S.A. and the USSR |journal=Perspect. Biol. Med. |volume=31 |issue=1 |pages=57–64 |year=1987 |pmid=3696960 |doi=10.1353/pbm.1987.0023}}</ref><ref>{{cite journal |author=Benison S |title=International medical cooperation: Dr. Albert Sabin, live poliovirus vaccine and the Soviets |journal=Bull Hist Med |volume=56 |issue=4 |pages=460–83 |year=1982 |pmid=6760938 }}</ref> Based on these results, the Sabin strains were chosen for worldwide distribution.<ref name = Sanofi>{{cite web | title = Competition to develop an oral vaccine | work = Conquering Polio | publisher = ] SA |date=2007-02-02 |url = http://www.polio.info/polio-eradication/front/templates/index.jsp?siteCode=POLIO&codeRubrique=34&lang=EN| archiveurl = https://web.archive.org/web/20071007095443/http://www.polio.info/polio-eradication/front/templates/index.jsp?siteCode=POLIO&codeRubrique=34&lang=EN | archivedate=2007-10-07}}</ref> | |||
| ] candy]] | |||
| There are 57 ] substitutions which distinguish the attenuated Sabin 1 strain from its virulent parent (the Mahoney serotype), two nucleotide substitutions attenuate the Sabin 2 strain, and 10 substitutions are involved in attenuating the Sabin 3 strain.<ref name=Kew_2005/> The primary attenuating factor common to all three Sabin vaccines is a mutation located in the virus's ] (IRES)<ref>{{cite journal |author=Ochs K |title=Impaired Binding of Standard Initiation Factors Mediates Poliovirus Translation Attenuation |journal=J. Virol. |volume=77 |issue=1 |pages=115–22 |date=January 2003 |pmid=12477816 |pmc=140626 |doi= 10.1128/JVI.77.1.115-122.2003|url=http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=12477816 |name-list-format=vanc|author2=Zeller A |author3=Saleh L |display-authors=3 |last4=Bassili |first4=G. |last5=Song |first5=Y. |last6=Sonntag |first6=A. |last7=Niepmann |first7=M.}}</ref> which alters ] structures, and reduces the ability of poliovirus to translate its RNA template within the host cell.<ref>{{cite journal |vauthors=Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E |title=Dual Stem Loops within the Poliovirus Internal Ribosomal Entry Site Control Neurovirulence |journal=J. Virol. |volume=73 |issue=2 |pages=958–64 |date=February 1999 |pmid=9882296 |pmc=103915 |url=http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=9882296}}</ref> The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of infection and replication, but is unable to replicate efficiently within ] tissue. | |||
| OPV is an ], produced by the passage of the virus through nonhuman cells at a sub] temperature, which produces spontaneous mutations in the viral genome.<ref name="Sabin_1960">{{cite journal|vauthors=Sabin AB, Ramos-Alvarez M, Alvarez-Amezquita J, Pelon W, Michaels RH, Spigland I, Koch MA, Barnes JM, Rhim JS|date=August 1960|title=Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses|journal=JAMA|volume=173|issue=14|pages=1521–1526|doi=10.1001/jama.1960.03020320001001|pmid=14440553}}</ref> Oral polio vaccines were developed by several groups, one of which was led by ]. Other groups, led by ] and ], developed their attenuated vaccine strains. In 1958, the ] created a special committee on live polio vaccines. The various vaccines were carefully evaluated for their ability to induce immunity to polio while retaining a low incidence of neuropathogenicity in monkeys. Large-scale clinical trials performed in the Soviet Union in the late 1950s to early 1960s by ] and his colleagues demonstrated the safety and high efficacy of the vaccine.<ref>{{cite journal | vauthors = Sabin AB | s2cid = 45655185 | title = Role of my cooperation with Soviet scientists in the elimination of polio: possible lessons for relations between the U.S.A. and the USSR | journal = Perspectives in Biology and Medicine | volume = 31 | issue = 1 | pages = 57–64 | year = 1987 | pmid = 3696960 | doi = 10.1353/pbm.1987.0023 }}</ref><ref>{{cite journal|vauthors=Benison S|year=1982|title=International medical cooperation: Dr. Albert Sabin, live poliovirus vaccine and the Soviets|journal=Bulletin of the History of Medicine|volume=56|issue=4|pages=460–483|pmid=6760938}}</ref> Based on these results, the Sabin strains were chosen for worldwide distribution.<ref name = Sanofi>{{cite web | title = Competition to develop an oral vaccine | work = Conquering Polio | publisher = ] SA |date=2 February 2007 |url = http://www.polio.info/polio-eradication/front/templates/index.jsp?siteCode=POLIO&codeRubrique=34&lang=EN| archive-url = https://web.archive.org/web/20071007095443/http://www.polio.info/polio-eradication/front/templates/index.jsp?siteCode=POLIO&codeRubrique=34&lang=EN | archive-date=7 October 2007}}</ref> Fifty-seven ] substitutions distinguish the attenuated Sabin 1 strain from its virulent parent (the Mahoney serotype), two nucleotide substitutions attenuate the Sabin 2 strain, and 10 substitutions are involved in attenuating the Sabin 3 strain.<ref name=Kew_2005/> The primary attenuating factor common to all three Sabin vaccines is a mutation located in the virus's ],<ref>{{cite journal|vauthors=Ochs K, Zeller A, Saleh L, Bassili G, Song Y, Sonntag A, Niepmann M|date=January 2003|title=Impaired binding of standard initiation factors mediates poliovirus translation attenuation|journal=Journal of Virology|volume=77|issue=1|pages=115–122|doi=10.1128/JVI.77.1.115-122.2003|pmc=140626|pmid=12477816}}</ref> which alters ] structures and reduces the ability of poliovirus to translate its RNA template within the host cell.<ref>{{cite journal|vauthors=Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E|date=February 1999|title=Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence|journal=Journal of Virology|volume=73|issue=2|pages=958–964|doi=10.1128/JVI.73.2.958-964.1999|pmc=103915|pmid=9882296}}</ref> The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of infection and replication, but is unable to replicate efficiently within ] tissue. In 1961, type 1 and 2 ] oral poliovirus vaccine (MOPV) was licensed, and in 1962, type 3 MOPV was licensed. In 1963, trivalent OPV (TOPV) was licensed, and became the vaccine of choice in the United States and most other countries of the world, largely replacing the inactivated polio vaccine.<ref name="Pearce">{{cite journal | vauthors = Pearce JM | title = Salk and Sabin: poliomyelitis immunisation | journal = Journal of Neurology, Neurosurgery, and Psychiatry | volume = 75 | issue = 11 | pages = 1552 | date = November 2004 | pmid = 15489385 | pmc = 1738787 | doi = 10.1136/jnnp.2003.028530 }}</ref> A second wave of mass immunizations led to a further dramatic decline in the number of polio cases. Between 1962 and 1965, about 100 million Americans (roughly 56% of the population at that time) received the Sabin vaccine. The result was a substantial reduction in the number of poliomyelitis cases, even from the much-reduced levels following the introduction of the Salk vaccine.<ref name=Smallman>{{cite book | vauthors = Smallman-Raynor M |title=Poliomyelitis: A World Geography: Emergence to Eradication |publisher=Oxford University Press (US) |year=2006 |isbn=978-0-19-924474-4|page= }}{{page needed|date=August 2021}}</ref> | |||
| In 1961, type 1 and 2 ] oral poliovirus vaccine (MOPV) was licensed, and in 1962, type 3 MOPV was licensed. In 1963, trivalent OPV (TOPV) was licensed, and became the vaccine of choice in the United States and most other countries of the world, largely replacing the inactivated polio vaccine.<ref name = Pearce/> A second wave of mass immunizations led to a further dramatic decline in the number of polio cases. Between 1962 and 1965 about 100 million Americans (roughly 56% of the population at that time) received the Sabin vaccine. The result was a substantial reduction in the number of poliomyelitis cases, even from the much reduced levels following the introduction of the Salk vaccine.<ref name=Smallman>{{cite book |author=Smallman-Raynor, Matthew |title=Poliomyelitis: A World Geography: Emergence to Eradication |publisher=Oxford University Press, USA |year=2006 |isbn=0-19-924474-X }}</ref> | |||
| OPV is usually provided in vials containing 10–20 doses of vaccine. A single dose of oral polio vaccine (usually two drops) contains 1,000,000 infectious units of Sabin 1 (effective against PV1), 100,000 infectious units of the Sabin 2 strain, and 600,000 infectious units of Sabin 3. The vaccine contains small traces of ]—] and ]—but does not contain ]s.<ref name=PAHO>{{cite book |title=Poliomyelitis Eradication: Field Guide |publisher=Pan American Health Organization |location=Washington |year=2006 |isbn=92-75-11607- |
OPV is usually provided in vials containing 10–20 doses of vaccine. A single dose of oral polio vaccine (usually two drops) contains 1,000,000 infectious units of Sabin 1 (effective against PV1), 100,000 infectious units of the Sabin 2 strain, and 600,000 infectious units of Sabin 3. The vaccine contains small traces of ]—] and ]—but does not contain ]s.<ref name=PAHO>{{cite book |title=Poliomyelitis Eradication: Field Guide |publisher=Pan American Health Organization |location=Washington |year=2006 |isbn=978-92-75-11607-4|page= }}{{page needed|date=August 2021}}</ref> | ||
| ==History== | ==History== | ||
| {{See also|Cold War tensions and the polio vaccine}} | |||
| In generic sense, vaccination works by priming the ] with an ']'. Stimulating immune response, via use of an infectious agent, is known as ]. The development of immunity to polio efficiently blocks person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and ].<ref name=Fine/> | |||
| In a generic sense, vaccination works by priming the ] with an ']'. Stimulating immune response, by use of an infectious agent, is known as ]. The development of immunity to polio efficiently blocks person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and ].<ref name=Fine/> | |||
| The development of two polio vaccines led to the first modern mass ]s. The last cases of paralytic poliomyelitis caused by endemic transmission of wild virus in the United States occurred in 1979, with an outbreak among the ] in several ] states.<ref name = PinkPages>{{cite book | |
The development of two polio vaccines led to the first modern mass ]s. The last cases of paralytic poliomyelitis caused by endemic transmission of wild virus in the United States occurred in 1979, with an outbreak among the ] in several ] states.<ref name = PinkPages>{{cite book | veditors = Atkinson W, Hamborsky J, McIntyre L, Wolfe S| title = Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) | edition = 10th ed. (2nd printing) | publisher = Public Health Foundation | location = Washington, D.C. | year = 2008 | url = https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio-508.pdf | access-date = 29 November 2008 |archive-url=https://web.archive.org/web/20080924184516/http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio-508.pdf |archive-date=24 September 2008}}</ref> | ||
| === |
=== 1930s === | ||
| In the 1930s, poliovirus was perceived as especially terrifying, as little was known of how the disease was transmitted or how it could be prevented. This virus was also notable for primarily impacting affluent children, making it a prime target for vaccine development, despite its relatively low mortality and morbidity.<ref name=":0">{{Cite journal| vauthors =Löwy I |date=1 April 2006|title=Book Review|journal=Medical History|volume=50|issue=2|pages=253–254|doi=10.1017/S0025727300009790|doi-broken-date=1 November 2024 |issn=0025-7273|pmc=1472109}}</ref> Despite this, the community of researchers in the field thus far had largely observed an informal moratorium on any vaccine development as it was perceived to present too high a risk for too little likelihood of success.<ref name=":1">{{cite book| vauthors = Rivers T |year=1967|title=Tom Rivers: reflections on a life in medicine and science: an oral history memoir|publisher=MIT Press|hdl=2027/heb.05734|isbn=978-0262020268|url=https://hdl.handle.net/2027/heb.05734}}</ref><ref name=":2">{{Cite book| vauthors = Halpern SA |title=Lesser harms: The morality of risk in medical research|publisher=University of Chicago Press|year=2006|location=Chicago, Ill|oclc=877210630}}</ref> | |||
| This year, two separate teams were at work developing and testing a polio vaccine. Both projects came to disastrous ends. At New York University, Maurice Brodie, MD (1903-1939), a young researcher, prepared a killed poliovirus vaccine, testing it on chimpanzees, on himself, and finally on children. He enrolled about 11,000 individuals (in both control and vaccine groups) in his trial. Meanwhile, John Kolmer, MD, of Temple University in Philadelphia developed an attenuated poliovirus vaccine, which he tested in about 10,000 children. | |||
| The tests proved a disaster. Several subjects died of polio, and many were paralyzed, made ill, or suffered allergic reactions to the vaccines.<ref>http://www.historyofvaccines.org/content/timelines/polio</ref> | |||
| This shifted in the early 1930s when American groups took up the challenge: ] led a team from the public health laboratory of the city of New York and ] collaborated with the ] in Philadelphia. The rivalry between these two researchers lent itself to a race-like mentality which, combined with a lack of oversight of medical studies, was reflected in the methodology and outcomes of each of these early vaccine development ventures.<ref name=":3">{{cite thesis|vauthors=Hovern D|title=The Trials and Triumphs of the American Polio Vaccine|year=2018|publisher=Cooper Medical School of Rowan University|url=https://rdw.rowan.edu/cmsru_capstones/4|access-date=19 July 2021|archive-date=21 July 2021|archive-url=https://web.archive.org/web/20210721042834/https://rdw.rowan.edu/cmsru_capstones/4/|url-status=live}}</ref><ref>{{Cite web|title=Vaccine Testing and Vulnerable Human Subjects {{!}} History of Vaccines|url=https://www.historyofvaccines.org/index.php/content/articles/vaccine-testing-and-vulnerable-human-subjects|access-date=18 July 2021|website=www.historyofvaccines.org|language=en|archive-date=2 August 2021|archive-url=https://web.archive.org/web/20210802132238/https://www.historyofvaccines.org/index.php/content/articles/vaccine-testing-and-vulnerable-human-subjects|url-status=dead}}</ref><ref name=":0" /> | |||
| === 1936 === | |||
| In 1936, Maurice Brodie, a research assistant at ], attempted to produce a ]-killed polio vaccine from ground-up monkey ]s. His initial attempts were hampered by the difficulty of obtaining enough virus. Brodie first tested the vaccine on himself and several of his assistants. He then gave the vaccine to three thousand children. Many of these children developed allergic reactions, but none developed immunity to polio.<ref name = Pearce>{{cite journal |author=Pearce J |title=Salk and Sabin: poliomyelitis immunisation |url= http://jnnp.bmj.com/cgi/content/full/75/11/1552 |journal=J Neurol Neurosurg Psychiatry |volume=75 |issue=11 |page=1552 |year=2004 |pmid = 15489385 |doi=10.1136/jnnp.2003.028530 |pmc=1738787}}</ref> Philadelphia pathologist John Kolmer also claimed to have developed a vaccine that same year, but it too produced no immunity and was blamed for causing cases of paralytic polio, nine of them fatal.<ref name=Rainsberger>{{cite web | author=Rainsberger M | title=More than a March of Dimes | publisher=The University of Texas at Austin | url=https://www.utexas.edu/features/2005/polio/index.html | date=2005-06-27 <!-- accessdate=2011-05-19 -->}}</ref> | |||
| === |
==== Kolmer's live vaccine ==== | ||
| Kolmer began his vaccine development project in 1932 and ultimately focused on producing an attenuated or ''live virus vaccine''. Inspired by the success of vaccines for rabies and yellow fever, he hoped to use a similar process to denature the polio virus.<ref name=":3" /> In order to go about ] his polio vaccine, he repeatedly passed the virus through monkeys.<ref name=":4">{{Cite book| vauthors = Gould T |url=http://www.worldcat.org/oclc/38243151|title=A Summer Plague: Polio and Its Survivors|year=1997|publisher=Yale University Press|isbn=978-0300072761|location=New Haven|oclc=38243151}}</ref> Using methods of production that were later described as "hair-raisingly amateurish, the therapeutic equivalent of bath-tub gin,"<ref>{{Cite book| vauthors = Wilson JR |title=Margin of Safety: The Story of Poliomyelitis Vaccine|publisher=Collins|year=1963|location=Garden City, NY|oclc=630735949}}</ref> Kolmer ground the spinal cords of his infected monkeys and soaked them in a salt solution. He then filtered the solution through mesh, treated it with ], and refrigerated the product for 14 days<ref name=":3" /> to ultimately create what would later be prominently critiqued as a "veritable witches brew".<ref>Paul, J.R. (1971). ''A History of Poliomyelitis''. New Haven: Yale University Press.{{ISBN?}}{{page needed|date=August 2021}}</ref> | |||
| A breakthrough came in 1948 when a research group headed by ] at the ] successfully cultivated the ] in human tissue in the laboratory.<ref name=Enders>{{cite journal |author=Enders JF, Weller TH, Robbins FC |title=Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues |journal=Science |volume=109 |issue=2822 |pages=85–87 |date=January 1949 |pmid=17794160 |doi=10.1126/science.109.2822.85 |url=http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=17794160|last2=Weller |last3=Robbins |bibcode=1949Sci...109...85E }}</ref> This group had recently successfully grown mumps in cell culture. In March 1948 ] was attempting to grow varicella virus in embryonic lung tissue. He had inoculated the planned number of tubes when he noticed that there were a few unused tubes. He retrieved a sample of mouse brain infected with polio virus and added it to the remaining test tubes, on the off chance that the virus might grow. The varicella cultures failed to grow but the polio cultures were successful. | |||
| This development greatly facilitated vaccine research and ultimately allowed for the development of vaccines against polio. Enders and his colleagues, ] and ], were recognized in 1954 for their labors with a ].<ref name=Nobel_1954>{{cite web | title=The Nobel Prize in Physiology or Medicine 1954 | publisher=The Nobel Foundation | url=http://nobelprize.org/nobel_prizes/medicine/laureates/1954/ | accessdate=2008-11-29}}</ref> Other important advances that led to the development of polio vaccines were: the identification of three poliovirus ]s (Poliovirus type 1 — PV1, or Mahoney; PV2, Lansing; and PV3, Leon); the finding that prior to paralysis, the virus must be present in the blood; and the demonstration that administration of antibodies in the form of ] protects against paralytic polio.<ref name="Kew_2005">{{cite journal |author=Kew O, Sutter R, de Gourville E, Dowdle W, Pallansch M |title=Vaccine-derived polioviruses and the endgame strategy for global polio eradication |journal=Annu Rev Microbiol |volume=59 |pages=587–635 |year=2005 |pmid=16153180 |doi=10.1146/annurev.micro.58.030603.123625 |url=http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.micro.58.030603.123625?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov|last2=Sutter |last3=De Gourville |last4=Dowdle |last5=Pallansch }}</ref><ref>{{cite journal |author=Hammon W, Coriell L, Wehrle P, Stokes J |title=Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. IV. Final report of results based on clinical diagnoses |journal=J Am Med Assoc |volume=151 |issue=15 |pages=1272–85 |year=1953 |pmid=13034471|last2=Coriell |last3=Wehrle |last4=Stokes Jr }}</ref> | |||
| ] (right) with ], circa 1985]] | |||
| In keeping with the norms of the time, Kolmer completed a relatively small animal trial with 42 monkeys before proceeding to ] in 1934.<ref name=":2" /> He tested his vaccine upon himself, his two children, and his assistant.<ref name=":2" /> He gave his vaccine to just 23 more children before declaring it safe and sending it out to doctors and health departments for a larger test of efficacy.<ref name=":2" /> By April 1935, he was able to report having tested the vaccine on 100 children without ill effect.<ref name=":5">{{Cite journal| vauthors = Berk LB |date=1989|title=Polio Vaccine Trials of 1935.|journal=Transactions & Studies of the College of Physicians of Philadelphia|volume=11|issue=4|pages=321–336|pmid=2692236}}</ref> Kolmer's first formal presentation of results would not come about until November 1935 where he presented the results of 446 children and adults he had vaccinated with his attenuated vaccine.<ref name=":5" /> He also reported that together the ] and the ] (the manufacturer who held the patent for his ricinoleating process) had distributed 12,000 doses of vaccine to some 700 physicians across the United States and Canada.<ref name=":5" /> Kolmer did not describe any monitoring of this experimental vaccination program nor did he provide these physicians with instructions in how to administer the vaccine or how to report side effects.<ref name=":5" /> Kolmer dedicated the bulk of his publications thereafter to explaining what he believed to be the cause of the 10+ reported cases of paralytic polio following vaccination, in many cases in towns where no polio outbreak had occurred.<ref name=":5" /><ref name="Offit_2007" /> Six of these cases had been fatal.<ref name=":5" /> Kolmer had no control group but asserted that many more children would have gotten sick.<ref name="Offit_2007" /> | |||
| === 1952–1953 === | |||
| The U.S. experienced an outbreak of 58,000 and 35,000 polio cases, respectively, up from a typical number of some 20,000 a year. Amid this U.S. polio epidemic, millions of dollars were invested in finding and marketing a polio vaccine by commercial interests, including Lederle Laboratories in New York under the direction of ]. Also working at Lederle was Polish-born ] and ] ], who claims to have created the first successful polio vaccine, in 1950. His vaccine, however, being a live attenuated virus taken orally, was still in the research stage and would not be ready for use until five years after Jonas Salk's polio vaccine (a dead-virus injectable vaccine) had reached the market. Koprowski's attenuated vaccine was prepared by successive passages through the brains of Swiss albino mice. By the seventh passage, the vaccine strains could no longer infect nervous tissue or cause paralysis. After one to three further passages on rats, the vaccine was deemed safe for human use.<ref name="Sanofi" /><ref>{{cite journal |title=Weekly Reports for OCTOBER 10, 1947 |journal=Public Health Rep |volume=62 |issue=41 |pages=1467–1498 |date=October 1947 |pmid=19316151 |pmc=1995293 |doi= }}</ref> On 27 February 1950, Koprowski's live, attenuated vaccine was tested for the first time on an 8-year-old boy living at ], an institution for the physically and mentally disabled located in New York. After the child suffered no side effects, Koprowski enlarged his experiment to include 19 other children.<ref name="Sanofi" /><ref>{{cite web|url=http://www.historyofvaccines.org/content/timelines/polio | title = Interview with Hilary Koprowski, sourced at History of Vaccines website | first=Hilary |last=Koprowski | date=15 October 2010 | accessdate=15 October 2010| publisher=]}}</ref> | |||
| ] during the early days of the National Polio Immunization Program.]] | |||
| ==== Brodie's inactivated vaccine ==== | |||
| ===Jonas Salk === | |||
| At nearly the same time as Kolmer's project, ] had joined immunologist ] at the ] where they worked together on poliovirus. With the aid of grant funding from the President's Birthday Ball Commission (a predecessor to what would become the ]), Brodie was able to pursue the development of an inactivated or "killed virus" vaccine. Brodie's process also began by grinding the spinal cords of infectious monkeys and then treating the cords with various germicides,<ref name=":6">{{Cite journal|vauthors=Broadie M, Park W|date=5 October 1935|title=Active immunization against poliomyelitis|url=http://ccat.sas.upenn.edu/goldenage/wonder/Archive/Science/wd_brodie%26park.htm|journal=Journal of the American Medical Association|volume=105|issue=14|pages=1089–1093|doi=10.1001/jama.1935.02760400005002|s2cid=1640997|access-date=19 July 2021|archive-date=19 July 2021|archive-url=https://web.archive.org/web/20210719142753/http://ccat.sas.upenn.edu/goldenage/wonder/Archive/Science/wd_brodie%26park.htm|url-status=live}}</ref> ultimately finding a solution of ] to be the most effective. By 1 June 1934, Brodie was able to publish his first scholarly article describing his successful induction of immunity in three monkeys with inactivated poliovirus.<ref>{{cite journal| vauthors = Brodie M |title=Active immunization in monkeys against poliomyelitis with germicidally inactivated virus|journal=The Journal of Immunology|volume=28|number=1|year=1935|pages=1–18|doi=10.4049/jimmunol.28.1.1 }}</ref><ref name=":7">{{Cite web|vauthors=Johnston K|date=22 February 2021|title=The tragic story of a Canadian vaccine trailblazer|url=https://www.macleans.ca/society/the-tragic-story-of-a-canadian-vaccine-trailblazer/|access-date=18 July 2021|website=Macleans.ca|language=en|archive-date=19 July 2021|archive-url=https://web.archive.org/web/20210719142802/https://www.macleans.ca/society/the-tragic-story-of-a-canadian-vaccine-trailblazer/|url-status=live}}</ref> Through continued study on an additional 26 monkeys, Brodie ultimately concluded that administration of live virus vaccine tended to result in ] while administration of killed virus vaccine tended to result in ].<ref name=":5" /> | |||
| ] himself, in 1957 at the ] where he and his team had developed the vaccine]] | |||
| The first effective polio vaccine was developed in 1952 by ] and a team at the ] that included ], Byron Bennett, L. James Lewis, and Lorraine Friedman, but it required years of subsequent testing. To encourage patience, Salk went on CBS radio to report a successful test on a small group of adults and children on 26 March 1953; two days later the results were published in '']''.<ref>{{cite book|last=Offit|first=Paul A.|title=The Cutter Incident: How America's First Polio Vaccine Led to the Growing Vaccine Crisis|publisher=Yale University Press|year=2007|page=38|isbn=0-300-12605-0}}</ref> Beginning 23 February 1954, the vaccine was tested at Arsenal Elementary School and the ] in ].<ref name="Shors2008">{{cite book|author=Teri Shors|title=Understanding viruses|url=https://books.google.com/books?id=VQeamKFwyvgC&pg=PA294|accessdate=22 February 2011|date=14 March 2008|publisher=Jones & Bartlett Learning|isbn=978-0-7637-2932-5|pages=294–}}</ref> Salk's vaccine was then used in a test called the Francis Field Trial, led by ]; the largest medical experiment in history. The test began with some 4,000 children at Franklin Sherman Elementary School in ],<ref name="test"> "Miracle Workers," ''American Heritage'', Winter 2010.</ref> and would eventually involve 1.8 million children, in 44 states from ] to ].<ref name=MoD_2004>{{cite web | title = Polio Victory Remembered as March of Dimes Marks 50th Anniversary of Salk Vaccine Field Trials | work = News Desk | url = http://www.marchofdimes.org/news/polio-victory-remembered-as-march-of-dimes-marks-50th-anniversary-of-salk-vaccine-field-trials.aspx | date = 2004-04-26 | accessdate = 2014-11-14}}</ref> By the conclusion of the study, roughly 440,000 received one or more injections of the vaccine, about 210,000 children received a ], consisting of harmless ], and 1.2 million children received no vaccination and served as a control group, who would then be observed to see if any contracted polio.<ref name = Sanofi/> The results of the field trial were announced 12 April 1955 (the tenth anniversary of the death of President ], whose ] was generally believed to have been caused by polio). The Salk vaccine had been 60–70% effective against PV1 (poliovirus type 1), over 90% effective against PV2 and PV3, and 94% effective against the development of bulbar polio.<ref name=Smith_1990>{{cite book |last = Smith | first = Jane S. | title = Patenting the Sun: Polio and the Salk Vaccine | publisher = William Morrow & Co |year= 1990 |isbn=0-688-09494-5}}</ref> Soon after Salk's vaccine was licensed in 1955, children's vaccination campaigns were launched. In the U.S, following a mass immunization campaign promoted by the ], the annual number of polio cases fell from 35,000 in 1953 to 5,600 by 1957.<ref name=Sass>{{cite book |vauthors=Sorem A, Sass EJ, Gottfried G |title=Polio's legacy: an oral history |publisher=University Press of America |location=Washington, D.C |year=1996 |isbn=0-7618-0144-8 |url=http://www.cloudnet.com/~edrbsass/poliotimeline.htm }}</ref>{{Unreliable medical source|date=September 2014}} By 1961 only 161 cases were recorded in the United States.<ref name=Hinman>{{cite journal |author=Hinman A |title=Landmark perspective: Mass vaccination against polio |journal=JAMA |volume=251 |issue=22 |pages=2994–6 |year=1984 |pmid = 6371280 |doi=10.1001/jama.1984.03340460072029}}</ref>] boy is injected with inactivated poliovirus vaccine (], 1993)]] | |||
| Soon after, following a similar protocol to Kolmer, Brodie proceeded with ] upon himself and his co-workers at the ] laboratory.<ref name=":2" /> Brodie's progress was eagerly covered by popular press as the public hoped for a successful vaccine to become available.<ref name=":7" /> Such reporting did not make mention of the 12 children in a New York City Asylum who were subjected to early safety trials.<ref name=":2" /> As none of the subjects experienced ill effects, Park, described by contemporaries as "never one to let grass grow under his feet,"<ref>{{cite book |vauthors=Oshinsky DM |title=Polio: An American Story |date=2005 |publisher=Oxford University Press |isbn=978-0-19-515294-4 |page=57 |url=https://books.google.com/books?id=p4YRDAAAQBAJ&pg=PA57 |language=en |access-date=23 March 2022 |archive-date=25 November 2023 |archive-url=https://web.archive.org/web/20231125140209/https://books.google.com/books?id=p4YRDAAAQBAJ&pg=PA57#v=onepage&q&f=false |url-status=live }}</ref> declared the vaccine safe.<ref name=":4" /> When a severe polio outbreak overwhelmed ], ] it became the first trial site for the new vaccine on very short notice. Between November 1934 - May 1935, over 1,500 doses of the vaccine were administered in Kern County. While initial results were very promising, insufficient staffing and poor protocol design left Brodie open to criticism when he published the California results in August 1935.<ref name=":7" /><ref name=":5" /> Through private physicians, Brodie also conducted a broader field study, including 9,000 children who received the vaccine and 4,500 age- and location-matched controls who did not receive a vaccine. Again, the results were promising. Of those who received the vaccine, only a few went on to develop polio. Most had been exposed before vaccination and none had received the full series of vaccine doses being studied.<ref name=":5" /> Additionally, a polio epidemic in ], ] provided an opportunity for the ] to conduct a highly structured trial of the Brodie vaccine using funding from the Birthday Ball Commission.<ref name=":4" /><ref name=":5" /> | |||
| ==== Academic reception ==== | |||
| While their work was ongoing, the larger community of ]s began to raise concerns regarding the safety and efficacy of the new poliovirus vaccines.<ref name=":1" /> At this time there was very little oversight of medical studies and the ethical treatment of study participants largely relied upon moral pressure from ] scientists.<ref name=":3" /> Brodie's inactivated vaccines faced scrutiny from many who felt killed virus vaccines could not be efficacious. While researchers were able to replicate the ] he had produced in his animal trials, the prevailing wisdom was that ] was essential for an efficacious vaccine.<ref name=":5" /> Kolmer directly questioned the killed virus approach in scholarly journals.<ref name=":6" /> Kolmer's studies however had raised even more concern with increasing reports of children becoming paralysed following vaccination with his live virus vaccine and notably, with paralysis beginning at the arm rather than the foot in many cases.<ref name=":8">{{cite journal | vauthors = | title = Poliomyelitis | journal = American Journal of Public Health and the Nation's Health | volume = 26 | issue = 2 | pages = 181–183 | date = February 1936 | pmid = 18014373 | pmc = 1562619 | doi = 10.2105/AJPH.26.2.181 }}</ref> Both Kolmer and Brodie were called to present their research at the Annual Meeting of the ] in ] WI in October 1935.<ref name=":1" /> Additionally, ] was asked to discuss each of the presented papers as a prominent critic of the vaccine development effort.<ref name=":1" /> This resulted in the ] arranging a Symposium on Poliomyelitis to be delivered at the Annual Meeting of their Southern Branch the following month.<ref name=":1" /> It was during the discussion at this meeting that James Leake of the ] stood to immediately present clinical evidence that the Kolmer vaccine had caused several deaths and then allegedly accused Kolmer of being a murderer.<ref name=":1" /> As Rivers recalled in his oral history, "All hell broke loose, and it seemed as if everybody was trying to talk at the same time...Jimmy Leake used the strongest language that I have ever heard used at a scientific meeting."<ref name=":1" /> In response to the attacks from all sides, Brodie was reported to have stood up and stated, "It looks as though, according to Dr. Rivers, my vaccine is no good, and, according to Dr. Leake, Dr Kolmer's is dangerous."<ref name=":1" /> Kolmer simply responded by stating, "Gentlemen, this is one time I wish the floor would open up and swallow me."<ref name=":1" /> Ultimately, Kolmer's live vaccine was undoubtedly shown to be dangerous and had already been withdrawn in September 1935 before the Milwaukee meeting.<ref name=":8" /><ref name="Offit_2007" /><ref name=":5" /> While the consensus of the symposium was largely skeptical of the efficacy of Brodie's vaccine, its safety was not in question and the recommendation was for a much larger well-controlled trial.<ref name=":8" /> However, when three children became ill with paralytic polio following a dose of the vaccine, the directors of the ] in Georgia (acting as the primary funders for the project) requested it be withdrawn in December 1935.<ref name=":8" /> Following its withdrawal, the previously observed moratorium on human poliomyelitis vaccine development resumed and there would not be another attempt for nearly 20 years.<ref name=":5" /><ref name="Offit_2007" /> | |||
| While Brodie had arguably made the most progress in the pursuit of a poliovirus vaccine, he suffered the most significant career repercussions due to his status as a less widely known researcher.<ref name=":7" /> Modern researchers recognize that Brodie may well have developed an effective polio vaccine, however, the basic science and technology of the time were insufficient to understand and utilize this breakthrough.<ref name=":5" /> Brodie's work using formalin-inactivated virus would later become the basis for the Salk vaccine, but he would not live to see this success.<ref name=":5" /> Brodie was fired from his position within three months of the symposium's publication.<ref name=":5" /> While he was able to find another laboratory position, he died of a heart attack only three years later at age 36.<ref name=":5" /><ref name=":7" /> By contrast, Park, who was believed in the community to be reaching senility at this point in his older age, was able to retire from his position with honors<ref name=":1" /> before he died in 1939.<ref name=":4" /> Kolmer, already an established and well-respected researcher, returned to ] as a professor of medicine.<ref name=":5" /> Kolmer had a very productive career, receiving multiple awards, and publishing countless papers, articles, and textbooks up until his retirement in 1957.<ref name=":4" /><ref name=":1" /><ref name="Offit_2007" /><ref>{{Cite web|url=http://www.aai.org/About/History/Past-Presidents-and-Officers/JohnAKolmer|title=John A. Kolmer, M.D.|access-date=28 November 2017|archive-date=1 December 2017|archive-url=https://web.archive.org/web/20171201033155/http://www.aai.org/About/History/Past-Presidents-and-Officers/JohnAKolmer|url-status=live}}</ref> | |||
| ===1948=== | |||
| A breakthrough came in 1948 when a research group headed by ] at the ] successfully cultivated the poliovirus in human tissue in the laboratory.<ref name="Enders">{{cite journal | vauthors = Enders JF, Weller TH, Robbins FC | title = Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues | journal = Science | volume = 109 | issue = 2822 | pages = 85–87 | date = January 1949 | pmid = 17794160 | doi = 10.1126/science.109.2822.85 | bibcode = 1949Sci...109...85E }}</ref> This group had recently successfully grown ] in ]. In March 1948, ] was attempting to grow ''varicella'' virus in embryonic lung tissue. He had inoculated the planned number of tubes when he noticed that there were a few unused tubes. He retrieved a sample of ] infected with poliovirus and added it to the remaining test tubes, on the off chance that the virus might grow. The ''varicella'' cultures failed to grow, but the polio cultures were successful. This development greatly facilitated vaccine research and ultimately allowed for the development of vaccines against polio. Enders and his colleagues, ] and ], were recognized in 1954 for their efforts with a ].<ref name="Nobel_1954">{{cite web | title=The Nobel Prize in Physiology or Medicine 1954 | publisher=The Nobel Foundation | url=http://nobelprize.org/nobel_prizes/medicine/laureates/1954/ | access-date=29 November 2008 |url-status = live| archive-url=https://web.archive.org/web/20081219224515/http://nobelprize.org/nobel_prizes/medicine/laureates/1954/ | archive-date=19 December 2008 | df=dmy-all }}</ref> Other important advances that led to the development of polio vaccines were: the identification of three poliovirus ]s (Poliovirus type 1 – PV1, or Mahoney; PV2, Lansing; and PV3, Leon); the finding that before paralysis, the virus must be present in the blood; and the demonstration that administration of antibodies in the form of ] protects against paralytic polio.<ref name=":1" /><ref name="Kew_2005">{{cite journal | vauthors = Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA | title = Vaccine-derived polioviruses and the endgame strategy for global polio eradication | journal = Annual Review of Microbiology | volume = 59 | pages = 587–635 | date = 2005 | pmid = 16153180 | doi = 10.1146/annurev.micro.58.030603.123625 | url = https://zenodo.org/record/1235033 | access-date = 30 June 2019 | archive-date = 9 July 2020 | archive-url = https://web.archive.org/web/20200709053801/https://zenodo.org/record/1235033 | url-status = live }}</ref><ref name="pmid13034471">{{cite journal|vauthors=Hammon WM, Coriell LL, Wehrle PF, Stokes J|date=April 1953|title=Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. IV. Final report of results based on clinical diagnoses|journal=Journal of the American Medical Association|volume=151|issue=15|pages=1272–1285|pmid=13034471}}</ref> | |||
| === 1950–1955 === | |||
| {{See also| Announcement of Polio vaccine success}} | |||
| ] | |||
| During the early 1950s, polio rates in the U.S. were above 25,000 annually; in 1952 and 1953, the U.S. experienced an outbreak of 58,000 and 35,000 polio cases, respectively, up from a typical number of some 20,000 a year, with deaths in those years numbering 3,200 and 1,400.<ref>{{cite journal | url = https://ourworldindata.org/polio | vauthors = Ochmann S, Roser M | date = 9 November 2017 | title = Polio – Polio Cases (OWID based on US Public Health Service (1910–1951) and US Center for Disease Control (1960–2010)) | journal = Our World in Data | access-date = 26 March 2018 | archive-date = 28 March 2018 | archive-url = https://web.archive.org/web/20180328105153/https://ourworldindata.org/polio | url-status = live }}</ref> Amid this U.S. polio epidemic, millions of dollars were invested in finding and marketing a polio vaccine by commercial interests, including Lederle Laboratories in New York under the direction of ]. Also working at Lederle was Polish-born ] and ] ] of the Wistar Institute in Philadelphia, who tested the first successful polio vaccine, in 1950.<ref name="Kowproski_obit"/><ref name=Kowproski5 /> His vaccine, however, being a live attenuated virus taken orally, was still in the research stage and would not be ready for use until five years after Jonas Salk's polio vaccine (a dead-virus injectable vaccine) had reached the market. Koprowski's attenuated vaccine was prepared by successive passages through the brains of Swiss albino mice. By the seventh passage, the vaccine strains could no longer infect nervous tissue or cause paralysis. After one to three further passages on rats, the vaccine was deemed safe for human use.<ref name="Sanofi" /><ref>{{cite journal|date=October 1947|title=Public Health Weekly Reports for October 10, 1947|journal=Public Health Reports|volume=62|issue=41|pages=1467–1498|pmc=1995293|pmid=19316151}}</ref> On 27 February 1950, Koprowski's live, attenuated vaccine was tested for the first time on an 8-year-old boy living at ], an institution for physically and mentally disabled people located in New York. After the child had no side effects, Koprowski enlarged his experiment to include 19 other children.<ref name="Sanofi" /><ref>{{cite web |url=http://www.historyofvaccines.org/content/timelines/polio |title=Interview with Hilary Koprowski, sourced at History of Vaccines website | vauthors = Koprowski H |date=15 October 2010 |access-date=15 October 2010 |publisher=] |url-status = live|archive-url=http://arquivo.pt/wayback/20160515175017/http://www.historyofvaccines.org/content/timelines/polio |archive-date=15 May 2016 }}</ref> | |||
| ==== Jonas Salk ==== | |||
| ] himself, in 1957 at the ], where his team had developed the vaccine]] | |||
| ]'' circa'' 1961 for the National Polio Immunization Program]] | |||
| The first effective polio vaccine was developed in 1952 by ] and a team at the ] that included ], Byron Bennett, L. James Lewis, and Lorraine Friedman, which required years of subsequent testing. Salk went on CBS radio to report a successful test on a small group of adults and children on 26 March 1953; two days later, the results were published in '']''.<ref name = "Offit_2007">{{cite book| vauthors = Offit PA |title=The Cutter Incident: How America's First Polio Vaccine Led to the Growing Vaccine Crisis|publisher=Yale University Press|year=2007|page=38|isbn=978-0-300-12605-1}}</ref> ] invented a key laboratory technique that enabled the mass production of the vaccine by a team she led in Toronto.<ref>{{Cite web|title=science.ca : Leone N. Farrell|url=http://www.science.ca/scientists/scientistprofile.php?pID=438|access-date=1 August 2021|website=www.science.ca|archive-date=28 June 2020|archive-url=https://web.archive.org/web/20200628213420/http://www.science.ca/scientists/scientistprofile.php?pID=438|url-status=live}}</ref><ref>{{cite web |vauthors=Elliott CK |title=Leone Norwood Farrell, PhD |url=http://www.polioplace.org/people/leone-norwood-farrell-phd |website=polioplace |publisher=Post-polio Health International |access-date=10 August 2019 |date=March 2011 |archive-date=9 August 2019 |archive-url=https://web.archive.org/web/20190809151030/http://www.polioplace.org/people/leone-norwood-farrell-phd |url-status=live }}</ref> Beginning 23 February 1954, the vaccine was tested at ] and the ] in ].<ref name="Shors2008">{{cite book| vauthors = Shors T |title=Understanding viruses |url= https://books.google.com/books?id=VQeamKFwyvgC&pg=PA294 |access-date=22 February 2011|date=2008|publisher=Jones & Bartlett Learning|isbn=978-0-7637-2932-5|pages=294–|url-status = live|archive-url=https://web.archive.org/web/20140721060240/http://books.google.com/books?id=VQeamKFwyvgC&pg=PA294|archive-date=21 July 2014}}</ref> | |||
| Salk's vaccine was then used in a test called the Francis Field Trial, led by ], the largest medical experiment in history at that time. The test began with about 4,000 children at Franklin Sherman Elementary School in ],<ref name="Conis">{{cite journal|vauthors=Conis E|title=Political Ills|journal=Distillations|date=2016|volume=2|issue=2|pages=34–37|url=https://www.sciencehistory.org/distillations/magazine/political-ills|access-date=27 March 2018|archive-date=20 March 2018|archive-url=https://web.archive.org/web/20180320230802/https://www.sciencehistory.org/distillations/magazine/political-ills|url-status=live}}</ref><ref name="test">{{cite web | url = http://www.americanheritage.com/content/miracle-workers | vauthors = Oshinsky D | title = Miracle Workers | work = American Heritage | date =Winter 2010 | access-date = 1 September 2014 | archive-date = 3 September 2014 | archive-url = https://web.archive.org/web/20140903073856/http://www.americanheritage.com/content/miracle-workers | url-status = live }}</ref> and eventually involved 1.8 million children, in 44 states from ] to ].<ref name=MoD_2004>{{cite web | title = Polio Victory Remembered as March of Dimes Marks 50th Anniversary of Salk Vaccine Field Trials | work = News Desk | url = http://www.marchofdimes.org/news/polio-victory-remembered-as-march-of-dimes-marks-50th-anniversary-of-salk-vaccine-field-trials.aspx | date = 26 April 2004 | access-date = 14 November 2014 |url-status = live| archive-url = https://web.archive.org/web/20150927112220/http://www.marchofdimes.org/news/polio-victory-remembered-as-march-of-dimes-marks-50th-anniversary-of-salk-vaccine-field-trials.aspx | archive-date = 27 September 2015 | df = dmy-all }}</ref> By the conclusion of the study, roughly 440,000 received one or more injections of the vaccine, about 210,000 children received a ], consisting of harmless ], and 1.2 million children received no vaccination and served as a control group, who would then be observed to see if any contracted polio.<ref name = Sanofi/> | |||
| The results of the field trial were announced on 12 April 1955 (the tenth anniversary of the death of President ], whose ] was generally believed to have been caused by polio). The Salk vaccine had been 60–70% effective against PV1 (poliovirus type 1), over 90% effective against PV2 and PV3, and 94% effective against the development of bulbar polio.<ref name="Smith_1990">{{cite book | vauthors = Smith JS | title = Patenting the Sun: Polio and the Salk Vaccine | publisher = William Morrow & Co | year = 1990 | isbn = 978-0-688-09494-2 | url-access = registration | url = https://archive.org/details/patentingsunpoli00smit }}</ref> Soon after Salk's vaccine was licensed in 1955, children's vaccination campaigns were launched. In the U.S., following a mass immunization campaign promoted by the ], the annual number of polio cases fell from 35,000 in 1953 to 5,600 by 1957.<ref name="Sass">{{cite book |vauthors=Sorem A, Sass EJ, Gottfried G |title=Polio's legacy: an oral history |publisher=University Press of America |location=Washington, D.C. |year=1996 |isbn=978-0-7618-0144-3}}</ref> By 1961 only 161 cases were recorded in the United States.<ref name="Hinman">{{cite journal|vauthors=Hinman AR|date=June 1984|title=Landmark perspective: Mass vaccination against polio|journal=JAMA|volume=251|issue=22|pages=2994–2996|doi=10.1001/jama.1984.03340460072029|pmid=6371280}}</ref> | |||
| A week before the announcement of the Francis Field Trial results in April 1955, ] at the ] in Paris had also announced an effective polio vaccine.<ref>The Pasteur Institute stated that an anti poliomyelitis vaccine, developed by Professor Pierre Lepine would soon be produced in large quantities. (Times, London, 4 April 1955).</ref><ref>{{cite web| work = Archives de l'Institut Pasteur |title=Pierre Lépine (1901–1989) – Notice biographique|url=http://webext.pasteur.fr/archives/e_lep0.html |access-date=28 November 2016|url-status = live|archive-url=https://web.archive.org/web/20161128194930/http://webext.pasteur.fr/archives/e_lep0.html|archive-date=28 November 2016}}</ref> | |||
| ====Safety incidents==== | |||
| In April 1955, soon after mass polio vaccination began in the US, the Surgeon General began to receive reports of patients who contracted paralytic polio about a week after being vaccinated with the Salk polio vaccine from the ] pharmaceutical company, with the paralysis starting in the limb the vaccine was injected into.<ref name="Jonas Salk: A Life">{{Cite book| vauthors = Jacobs CD |title=Jonas Salk: A Life|publisher=Oxford University Press|year=2015|location=New York, NY|oclc=919967059}}</ref> The Cutter vaccine had been used in vaccinating 409,000 children in the western and midwestern United States.<ref>{{cite journal|date=August 1955|title=Technical Report on Poliomyelitis Vaccine|journal=Public Health Reports|volume=70|issue=8|pages=742}}</ref> | |||
| Later investigations showed that the Cutter vaccine had caused 260 cases of polio, killing 11.<ref name="Jonas Salk: A Life"/> | |||
| In response, the Surgeon General pulled all polio vaccines made by Cutter Laboratories from the market, but not before 260 cases of paralytic illness had occurred. Eli Lilly, Parke-Davis, Pitman-Moore, and Wyeth polio vaccines were also reported to have paralyzed numerous children. It was soon discovered that some lots of Salk polio vaccine made by Cutter, Wyeth, and the other labs had not been properly inactivated, allowing live poliovirus into more than 100,000 doses of vaccine. In May 1955, the National Institutes of Health and Public Health Services established a Technical Committee on Poliomyelitis Vaccine to test and review all polio vaccine lots and advise the Public Health Service as to which lots should be released for public use. These incidents reduced public confidence in the polio vaccine, leading to a drop in vaccination rates.<ref name="Juskewitch2010">{{Cite journal |vauthors=Juskewitch JE, Tapia CJ, Windebank AJ |date=August 2010 |title=Lessons from the Salk polio vaccine: methods for and risks of rapid translation |journal=Clinical and Translational Science |type=Review |volume=3 |issue=4 |pages=182–185 |doi=10.1111/j.1752-8062.2010.00205.x |pmc=2928990 |pmid=20718820 |doi-access=free}}</ref> | |||
| ===1961=== | |||
| ] (right) with ], ''circa'' 1985]] | |||
| At the same time that Salk was testing his vaccine, both ] and Hilary Koprowski continued working on developing a vaccine using live virus. During a meeting in Stockholm to discuss polio vaccines in November 1955, Sabin presented results obtained on a group of 80 volunteers, while Koprowski read a paper detailing the findings of a trial enrolling 150 people.<ref name=Sanofi/> Sabin and Koprowski both eventually succeeded in developing vaccines. Because of the commitment to the Salk vaccine in America, Sabin and Koprowski both did their testing outside the United States, Sabin in Mexico<ref name = "Sabin_1960" /> and the Soviet Union,<ref name=NMAH>{{cite web | url = http://amhistory.si.edu/polio/virusvaccine/vacraces2.htm | title = Two Vaccines: Sabin and Salk | work = Smithsonian National Museum of American History | date = 27 September 2021 | access-date = 24 April 2017 | archive-date = 20 January 2017 | archive-url = https://web.archive.org/web/20170120113212/http://amhistory.si.edu/polio/virusvaccine/vacraces2.htm | url-status = live }}</ref> Koprowski in the Congo and Poland.<ref name=Kowproski5/> In 1957, Sabin developed a trivalent vaccine containing attenuated strains of all three types of poliovirus.<ref name=NMAH /> In 1959, ten million children in the ] received the Sabin oral vaccine. For this work, Sabin was given the medal of the ], described as the Soviet Union's highest civilian honor.<ref>{{cite web | work = U.P.I. archives | url = http://www.upi.com/Archives/1986/11/20/Sabin-receives-highest-Soviet-civilian-honor/6402532846800/ | title = Sabin receives highest Soviet civilian honor | date = 20 November 1986 | access-date = 25 April 2017 | archive-date = 25 April 2017 | archive-url = https://web.archive.org/web/20170425122629/http://www.upi.com/Archives/1986/11/20/Sabin-receives-highest-Soviet-civilian-honor/6402532846800/ | url-status = live }}</ref> Sabin's oral vaccine using live virus came into commercial use in 1961.<ref name=WHO2016/> | |||
| Once Sabin's oral vaccine became widely available, it supplanted Salk's injected vaccine, which had been tarnished in the public's opinion by the ] of 1955, in which Salk vaccines improperly prepared by one company resulted in several children dying or becoming paralyzed.<ref name = "Offit_2007" /> | |||
| === 1987 === | === 1987 === | ||
| An enhanced-] IPV |
An enhanced-] IPV was licensed in the United States in November 1987, and is currently the vaccine of choice there.<ref name = PinkPages/> The first dose of the polio vaccine is given shortly after birth, usually between 1 and 2 months of age, and a second dose is given at 4 months of age.<ref name=PinkPages/> The timing of the third dose depends on the vaccine formulation but should be given between 6 and 18 months of age.<ref name=UK/> A booster vaccination is given at 4 to 6 years of age, for a total of four doses at or before school entry.<ref name="Peds">{{cite journal|date=February 1997|title=Poliomyelitis prevention: recommendations for use of inactivated poliovirus vaccine and live oral poliovirus vaccine. American Academy of Pediatrics Committee on Infectious Diseases|journal=Pediatrics|volume=99|issue=2|pages=300–305|doi=10.1542/peds.99.2.300|pmid=9024465|doi-access=free}}</ref> In some countries, a fifth vaccination is given during ].<ref name=UK/> Routine vaccination of adults (18 years of age and older) in developed countries is neither necessary nor recommended because most adults are already immune and have a very small risk of exposure to wild poliovirus in their home countries.<ref name=PinkPages/> In 2002, a ] (five-component) combination vaccine (called Pediarix)<ref>Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus Vaccine Combined</ref><ref name=pediarix>{{cite web |title=Pediarix |url=https://www.fda.gov/vaccines-blood-biologics/vaccines/pediarix |website=U.S. ] (FDA) |date=6 November 2019 |access-date=8 July 2020 |archive-date=22 September 2019 |archive-url=https://web.archive.org/web/20190922202657/https://www.fda.gov/vaccines-blood-biologics/vaccines/pediarix |url-status=live }}</ref> containing IPV was approved for use in the United States.<ref>{{cite web |url=https://www.fda.gov/media/75250/download |format=PDF |title=FDA Statistical Review and Evaluation |website=U.S. ] (FDA) |access-date=8 July 2020 |archive-date=6 August 2020 |archive-url=https://web.archive.org/web/20200806060853/https://www.fda.gov/media/75250/download |url-status=live }}</ref><ref name=pediarix/> | ||
| ===1988=== | ===1988=== | ||
| ] boy is injected with inactivated poliovirus vaccine (], 1993)]] | |||
| A global effort to eradicate polio, led by the ], ],<ref>{{cite news|last1=Mansoor|first1=Halima|title=Can India's social mobilisation strategy work in Pakistan?|url=http://tribune.com.pk/story/978251/can-indias-social-mobilisation-strategy-work-in-pakistan/|accessdate=24 October 2015|publisher=The Express Tribune|date=23 October 2015}}</ref> and ], began in 1988 and has relied largely on the oral polio vaccine developed by ] and ] (Sabin-Chumakov vaccine).<ref name=Watch>{{cite web| last = Mastny| first = Lisa | title = Eradicating Polio: A Model for International Cooperation |publisher = Worldwatch Institute | date = 1999-01-25 | url = http://www.worldwatch.org/node/1644 | accessdate = 2008-11-29}}</ref> | |||
| A global effort to eradicate polio, led by the ] (WHO), ],<ref>{{cite news| vauthors = Mansoor H |title=Can India's social mobilisation strategy work in Pakistan?|url=http://tribune.com.pk/story/978251/can-indias-social-mobilisation-strategy-work-in-pakistan/|access-date=24 October 2015|newspaper=The Express Tribune|date=23 October 2015|url-status = live|archive-url=https://web.archive.org/web/20151024053606/http://tribune.com.pk/story/978251/can-indias-social-mobilisation-strategy-work-in-pakistan/|archive-date=24 October 2015}}</ref> and the ], began in 1988, and has relied largely on the oral polio vaccine developed by ] and ] (Sabin-Chumakov vaccine).<ref name=Watch>{{cite web | vauthors = Mastny L | title = Eradicating Polio: A Model for International Cooperation | publisher = Worldwatch Institute | date = 25 January 1999 | url = http://www.worldwatch.org/node/1644 | access-date = 29 November 2008 |url-status = live| archive-url = https://web.archive.org/web/20061203222228/https://www.worldwatch.org/node/1644 | archive-date = 3 December 2006 | df = dmy-all }}</ref> | |||
| === Post-1990 === | |||
| Polio was eliminated in the ] by 1994.<ref name=MMWR_1994>{{cite journal | title = International Notes Certification of Poliomyelitis Eradication — the Americas, 1994 | journal = Morbidity and Mortality Weekly Report | publisher = Centers for Disease Control and Prevention | volume= 43 | issue= 39 | pages = 720–722 | year=1994 | url = http://www.cdc.gov/mmwr/preview/mmwrhtml/00032760.htm | pmid = 7522302| author1= Centers for Disease Control and Prevention (CDC) }}</ref> The disease was officially eliminated in 36 Western Pacific countries, including China and Australia in 2000.<ref name=Pacific>{{cite journal | author = ,| title = General News. Major Milestone reached in Global Polio Eradication: Western Pacific Region is certified Polio-Free | journal = Health Educ Res | year = 2001 | volume = 16 | issue = 1 | pages = 109–114 | url= http://her.oxfordjournals.org/cgi/reprint/16/1/109.pdf | format = PDF| doi = 10.1093/her/16.1.109}}</ref><ref name="D'Souza_2002">{{cite journal |author=D'Souza R, Kennett M, Watson C |title=Australia declared polio free |journal=Commun Dis Intell |volume=26 |issue=2 |pages=253–60 |year=2002 |pmid=12206379|last2=Kennett |last3=Watson }}</ref> ] was declared polio-free in 2002.<ref name=WHO_Europe_2002>{{cite press release | title = Europe achieves historic milestone as Region is declared polio-free | publisher = European Region of the World Health Organization | date = 2002-06-21 | url = http://www.who.int/mediacentre/news/releases/releaseeuro02/en/index.html | accessdate = 2008-08-23 }}</ref> Since January 2011, there were no reported cases of the disease in India, and hence in February 2012, the country was taken off the WHO list of polio endemic countries. If there are no cases of polio in the country for two more years, it will be declared as a polio-free country.<ref>{{cite news|last=Ray|first=Kalyan|title=India wins battle against dreaded polio|newspaper=Deccan Herald|date=26 February 2012}}</ref><ref>{{cite news|title=India polio-free for a year: 'First time in history we're able to put up such a map'|url=http://www.telegraphindia.com/1120226/jsp/frontpage/story_15181357.jsp|accessdate=26 February 2012|newspaper=The Telegraph|date=26 February 2012}}</ref> As of 2016, polio remains ] in only two countries: ], and ].{{cn|date=July 2016}} Although poliovirus transmission has been interrupted in much of the world, transmission of wild poliovirus does continue and creates an ongoing risk for the importation of wild poliovirus into previously polio-free regions. If importations of poliovirus occur, outbreaks of poliomyelitis may develop, especially in areas with low vaccination coverage and poor sanitation. As a result, high levels of vaccination coverage must be maintained.<ref name=MMWR_1994/> In November 2013, the ] announced a polio outbreak in Syria. In response, the ]n government put out a notice asking ] under age 15 to get the polio vaccine.<ref>Lisa Barron, , ''CISTran Finance'', 4 Nov 2013. Retrieved 18 Dec 2013.</ref> As of 2014, polio virus has spread out to ten countries mainly in ], ] and the ] with Pakistan, ] and ] advising vaccinations to outbound travelers.<ref name="DarrasJr.2014">{{cite book|author1=Basil T. Darras|author2=H. Royden Jones, Jr. Jr.|author3=Monique M. Ryan|author4=Darryl C. De Vivo|title=Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician's Approach|url=https://books.google.com/books?id=JlECBAAAQBAJ&pg=PA161|year=2014|publisher=Elsevier Science|isbn=978-0-12-417127-5|page=161}}</ref> In 2015, the World Health Organization announced a deal with the ] to encourage them to distribute the vaccine in areas they control.<ref>{{cite web|url=http://www.bloomberg.com/news/articles/2015-12-15/taliban-join-global-effort-to-kill-off-polio-in-2016|title=Taliban Join Global Effort to Kill Off Polio in 2016|author=Eltaf Najafizada|date=15 December 2015|work=Bloomberg.com}}</ref> Polio vaccination programs have received resistance from some people in Pakistan, Afghanistan, and Nigeria (the three countries with remaining polio cases). Indeed, on September 11, 2016, two unidentified gunmen associated with the Pakistani Taliban, Jamaat-ul-Ahrar, shot Zakaullah Khan, a doctor who was administering polio vaccines in Pakistan. The leader of the Jamaat-ul-Ahrar claimed responsibility for the shooting and says that the group will continue to keep doing these kinds of attacks. There is a history surrounding vaccines in Pakistan as in 2011 the CIA organized a fake vaccination program in order to help find ]. Additionally, some people believe that the vaccines are secretly being used for sterilization of Muslims. This resistance to and skepticism of vaccinations has consequently slowed down the polio eradication process within the three remaining countries. <ref>{{cite news|title=Pakistan polio official killed in Peshawar: police|url=https://www.dailystar.com.lb/News/World/2016/Sep-11/371570-pakistan-polio-official-killed-in-peshawar-police.ashx|accessdate=11 September 2016|publisher=The Daily Star: Lebanon|date=September 11, 2016}}</ref> | |||
| ===After 1990=== | |||
| Polio was eliminated in the Americas by 1994.<ref name="MMWR_1994">{{cite journal | title = Certification of poliomyelitis eradication--the Americas, 1994 | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 43 | issue = 39 | pages = 720–722 | date = October 1994 | pmid = 7522302 | url = https://www.cdc.gov/mmwr/preview/mmwrhtml/00032760.htm | url-status = live | publisher = Centers for Disease Control and Prevention | archive-url = https://web.archive.org/web/20170521163051/https://www.cdc.gov/mmwr/preview/mmwrhtml/00032760.htm | archive-date = 21 May 2017 | author1 = Centers for Disease Control Prevention (CDC) }}</ref> The disease was officially eliminated in 36 Western Pacific countries, including China and Australia, in 2000.<ref name="Pacific">{{cite journal|year=2001|title=General News. Major Milestone reached in Global Polio Eradication: Western Pacific Region is certified Polio-Free|journal=Health Educ Res|volume=16|issue=1|pages=109–114|bibcode=2001PDiff..16..110Y|doi=10.1093/her/16.1.109| vauthors = Yamazaki S, Toraya H }}</ref><ref name="D'Souza_2002">{{cite journal | vauthors = D' Souza RM, Kennett M, Watson C | title = Australia declared polio-free | journal = Communicable Diseases Intelligence Quarterly Report | volume = 26 | issue = 2 | pages = 253–260 | date = 2002 | pmid = 12206379 }}</ref> Europe was declared polio-free in 2002.<ref name=WHO_Europe_2002>{{cite press release | title = Europe achieves historic milestone as Region is declared polio-free | publisher = ] (WHO) | date = 21 June 2002 | url =https://www.who.int/mediacentre/news/releases/releaseeuro02/en/index.html | access-date = 23 August 2008 |url-status = dead| archive-url = https://web.archive.org/web/20080916065107/http://www.who.int/mediacentre/news/releases/releaseeuro02/en/index.html | archive-date = 16 September 2008 | df = dmy-all }}</ref> Since January 2011, no cases of the disease have been reported in India, hence in February 2012, the country was taken off the WHO list of polio-endemic countries. In March 2014, India was declared a polio-free country.<ref>{{cite news| vauthors = Ray K |title=India wins battle against dreaded polio|newspaper=Deccan Herald|date=26 February 2012}}</ref><ref>{{cite news |title=India polio-free for a year: 'First time in history we're able to put up such a map' |url= http://www.telegraphindia.com/1120226/jsp/frontpage/story_15181357.jsp |access-date=26 February 2012 |newspaper=The Telegraph |date=26 February 2012 |url-status = dead|archive-url=https://web.archive.org/web/20120227220101/http://www.telegraphindia.com/1120226/jsp/frontpage/story_15181357.jsp|archive-date=27 February 2012 }}</ref><ref>{{Cite web|url=http://www.searo.who.int/entity/immunization/topics/polio/en/|title=India three years polio-free|website=] (WHO)|access-date=19 February 2017|url-status = dead|archive-url=https://web.archive.org/web/20170301051406/http://www.searo.who.int/entity/immunization/topics/polio/en/|archive-date=1 March 2017}}</ref> | |||
| Although poliovirus transmission has been interrupted in much of the world, transmission of wild poliovirus does continue and creates an ongoing risk for the importation of wild poliovirus into previously polio-free regions. If importations of poliovirus occur, outbreaks of poliomyelitis may develop, especially in areas with low vaccination coverage and poor sanitation. As a result, high levels of vaccination coverage must be maintained.<ref name=MMWR_1994/> In November 2013, the WHO announced a polio outbreak in Syria. In response, the Armenian government put out a notice asking ] under age 15 to get the polio vaccine.<ref>{{cite web | vauthors = Barron L | url = http://cistranfinance.com/news/armenian-health-ministry-urges-syrian-armenian-children-to-get-polio-vaccine/666/ | title = Armenian Health Ministry: Syrian Armenian children need polio vaccine | archive-url = https://web.archive.org/web/20131219024041/http://cistranfinance.com/news/armenian-health-ministry-urges-syrian-armenian-children-to-get-polio-vaccine/666/ | archive-date=19 December 2013 | work = CISTran Finance | date = 4 November 2013 | access-date = 18 December 2013 }}</ref> As of 2014, polio virus had spread to 10 countries, mainly in Africa, Asia, and the ], with Pakistan, Syria, and ] advising vaccinations to outbound travellers.<ref name="DarrasJr.2014">{{cite book| vauthors = De Vivo DC, Ryan MM, Jones HR, Darras BT |title=Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician's Approach|url=https://books.google.com/books?id=JlECBAAAQBAJ&pg=PA161|year=2014|publisher=Elsevier Science|isbn=978-0-12-417127-5|page=161|url-status = live|archive-url=https://web.archive.org/web/20170423222313/https://books.google.com/books?id=JlECBAAAQBAJ&pg=PA161|archive-date=23 April 2017}}</ref> | |||
| Polio vaccination programs have been resisted by some people in Pakistan, Afghanistan, and Nigeria - the three countries as of 2017 with remaining polio cases. Almost all Muslim religious and political leaders have endorsed the vaccine,<ref>{{cite book|title=The Missing Martyrs|author=Charles Kurzman|publisher=]|page=130|quote=Resistance to polio vaccination is a fringe position. Almost every Muslim scholar and political leader has endorsed the vaccine, and all but a few Muslim-majority countries have wiped out the disease entirely.}}</ref> but a fringe minority believes that the vaccines are secretly being used for the sterilisation of Muslims.<ref name="Jegede">{{cite journal | vauthors = Jegede AS | title = What led to the Nigerian boycott of the polio vaccination campaign? | journal = PLOS Medicine | volume = 4 | issue = 3 | pages = e73 | date = March 2007 | pmid = 17388657 | pmc = 1831725 | doi = 10.1371/journal.pmed.0040073 | doi-access = free }}</ref> The fact that the ] to help find ] is an additional cause of distrust.<ref name="Pakistan">{{cite news|date=11 September 2016|title=Pakistan polio official killed in Peshawar: police|publisher=The Daily Star: Lebanon|url=https://www.dailystar.com.lb/News/World/2016/Sep-11/371570-pakistan-polio-official-killed-in-peshawar-police.ashx|url-status=live|access-date=11 September 2016|archive-url=https://web.archive.org/web/20160911122228/http://www.dailystar.com.lb/News/World/2016/Sep-11/371570-pakistan-polio-official-killed-in-peshawar-police.ashx|archive-date=11 September 2016}}</ref> In 2015, the WHO announced a deal with the ] to encourage them to distribute the vaccine in areas they control.<ref>{{cite news|url=https://www.bloomberg.com/news/articles/2015-12-15/taliban-join-global-effort-to-kill-off-polio-in-2016|title=Taliban Join Global Effort to Kill Off Polio in 2016| vauthors = Najafizada E |date=15 December 2015|work=Bloomberg.com|url-status = live|archive-url=https://web.archive.org/web/20170228052344/https://www.bloomberg.com/news/articles/2015-12-15/taliban-join-global-effort-to-kill-off-polio-in-2016|archive-date=28 February 2017}}</ref> However, the Pakistani Taliban was not supportive. On 11 September 2016, two unidentified gunmen associated with the Pakistani Taliban, Jamaat-ul-Ahrar, shot Zakaullah Khan, a doctor who was administering polio vaccines in Pakistan. The leader of the Jamaat-ul-Ahrar claimed responsibility for the shooting and stated that the group would continue this type of attack. Such resistance to and skepticism of vaccinations has consequently slowed down the polio eradication process within the two remaining endemic countries.<ref name="Pakistan" /> | |||
| == Travel requirements == | |||
| [[File:Polio vaccination travel requirements map.svg|thumb|400px|Polio vaccination is required for travellers... | |||
| {{Legend|#000080|From all countries, to all countries}} | |||
| {{Legend|#0000FF|From some countries, to all countries}} | |||
| {{Legend|#800080|From some countries, to all countries}} | |||
| {{Legend|#008000|From some countries, to some countries}} | |||
| {{Legend|#87DE87|To some countries}} | |||
| {{Legend|#FFCC00|From some countries}}]] | |||
| {{See also|Vaccination requirements for international travel}} | |||
| Travellers who wish to enter or leave certain countries must be vaccinated against polio, usually at most 12 months and at least 4 weeks before crossing the border, and be able to present a vaccination record/certificate at the border checks.<ref name="WHO Travel and Health">{{Cite web |url=https://www.who.int/ith/CHAPTER_6_For_Publication.pdf |title=International Travel and Health. Chapter 6 – Vaccine-preventable diseases and vaccines (2019 update) |work=World Health Organization |publisher=United Nations |date=2020 |access-date=2 December 2020 |archive-date=11 April 2020 |archive-url=https://web.archive.org/web/20200411151558/https://www.who.int/ith/CHAPTER_6_For_Publication.pdf |url-status=live }}</ref>{{rp|25–27}} Most requirements apply only to travel to or from so-called 'polio-endemic', 'polio-affected', 'polio-exporting', 'polio-transmission', or 'high-risk' countries.<ref name="WHO country list">{{Cite web |url=https://www.who.int/publications/m/item/countries-with-risk-of-yellow-fever-transmission-and-countries-requiring-yellow-fever-vaccination-(july-2019) |title=Countries with risk of yellow fever transmission and countries requiring yellow fever vaccination (July 2019) |work=World Health Organization |publisher=United Nations |date=4 July 2019 |access-date=2 December 2020 |archive-date=27 January 2021 |archive-url=https://web.archive.org/web/20210127232029/https://www.who.int/publications/m/item/countries-with-risk-of-yellow-fever-transmission-and-countries-requiring-yellow-fever-vaccination-(july-2019) |url-status=live }}</ref> As of August 2020, Afghanistan and Pakistan are the only polio-endemic countries in the world (where ]).<ref name=":9">{{Cite news| vauthors = Scherbel-Ball N |date=25 August 2020|title=Africa declared free of polio|language=en-GB|work=BBC News|url=https://www.bbc.com/news/world-africa-53887947|access-date=25 August 2020|archive-date=26 August 2020|archive-url=https://web.archive.org/web/20200826154956/https://www.bbc.com/news/world-africa-53887947|url-status=live}}</ref> Several countries have additional precautionary polio vaccination travel requirements, for example to and from 'key at-risk countries', which as of December 2020 include China, Indonesia, Mozambique, Myanmar, and Papua New Guinea.<ref name="WHO country list"/><ref>{{Cite news |url=http://polioeradication.org/where-we-work/key-at-risk-countries/ |title=Key At-Risk Countries |work=Global Polio Eradication Initiative |publisher=World Health Organization |access-date=2 December 2020 |archive-date=29 April 2021 |archive-url=https://web.archive.org/web/20210429061349/https://polioeradication.org/where-we-work/key-at-risk-countries/ |url-status=live }}</ref> | |||
| {| class="wikitable sortable" style="text-align:left;" align="left" width=1100 | |||
| ! colspan="2" align="left" | Polio vaccination requirements for international travel<ref name="WHO country list"/> | |||
| |- | |||
| ! align="left" width=100 | Country | |||
| ! Details | |||
| |- | |||
| | {{flag|Afghanistan}} | |||
| | Travellers from polio-endemic countries (Pakistan) need ] proof of polio vaccination (received between 4 weeks and 12 months before departure) upon arrival. Residents and all travellers staying in Afghanistan longer than 4 weeks need proof of polio vaccination (received between 4 weeks and 12 months before departure) when departing from Afghanistan.<ref name="WHO country list"/><ref>{{Cite web |url=https://www.iamat.org/country/afghanistan/risk/polio |title=Afghanistan Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=1 October 2020 |access-date=2 December 2020 |archive-date=21 October 2020 |archive-url=https://web.archive.org/web/20201021023033/https://www.iamat.org/country/afghanistan/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Belize}} | |||
| | Travellers from Afghanistan and Pakistan need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Belize residents travelling to countries with confirmed polio cases also need proof of vaccination.<ref>{{Cite web |url=https://www.iamat.org/country/belize/risk/polio |title=Belize Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030223600/https://www.iamat.org/country/belize/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Brunei}} | |||
| | Travellers from polio-exporting countries need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/brunei-darussalam/risk/polio |title=Brunei Darussalam Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=1 December 2020 |archive-url=https://web.archive.org/web/20201201114654/https://www.iamat.org/country/brunei-darussalam/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Egypt}} | |||
| | Travellers from Afghanistan, Angola, Benin, Cameroon, the Central African Republic, China, Congo-Kinshasa, Ethiopia, Ghana, Indonesia, Kenya, Mozambique, Myanmar, Niger, Nigeria, Pakistan, Papua New Guinea, Philippines, and Somalia need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/egypt/risk/polio |title=Egypt Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=8 March 2021 |archive-url=https://web.archive.org/web/20210308061617/https://www.iamat.org/country/egypt/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Georgia}} | |||
| | Travellers from at-risk countries need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Travellers without proof are offered OPV vaccination upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/georgia/risk/polio |title=Georgia Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=17 January 2021 |archive-url=https://web.archive.org/web/20210117032629/https://www.iamat.org/country/georgia/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|India}} | |||
| | Travellers from Afghanistan, Congo-Kinshasa, Ethiopia, Kenya, Nigeria, Pakistan, Somalia, and Syria need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/india/risk/polio |title=India Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030230943/https://www.iamat.org/country/india/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Iran}} | |||
| | Travellers from Afghanistan, Pakistan, and Nigeria need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Travellers without proof will be vaccinated upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/iran/risk/polio |title=Iran Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030075425/https://www.iamat.org/country/iran/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Iraq}} | |||
| | Travellers aged 15+ from Afghanistan and Pakistan need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival; children under age 15 must have received three doses of polio vaccine before travel. Travellers without proof will be vaccinated upon arrival. Travellers departing from Iraq to Afghanistan and Pakistan must also provide proof of vaccination upon departure.<ref>{{Cite web |url=https://www.iamat.org/country/iraq/risk/polio |title=Iraq Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=20 January 2021 |archive-url=https://web.archive.org/web/20210120224455/https://www.iamat.org/country/iraq/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Jordan}} | |||
| | Travellers from Afghanistan and Pakistan need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/jordan/risk/polio |title=Jordan Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030073452/https://www.iamat.org/country/jordan/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Lebanon}} | |||
| | Travellers from and to polio-affected countries need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/lebanon/risk/polio |title=Lebanon Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030213817/https://www.iamat.org/country/lebanon/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Libya}} | |||
| | Travellers from Afghanistan and Pakistan need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/libya/risk/polio |title=Libya Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030221107/https://www.iamat.org/country/libya/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Maldives}} | |||
| | Travellers from and to polio-exporting countries, as well as ] and ] pilgrims, need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/maldives/risk/polio |title=Maldives Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030083714/https://www.iamat.org/country/maldives/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Morocco}} | |||
| | Travellers from polio-affected countries need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/morocco/risk/polio |title=Morocco Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=21 October 2020 |archive-url=https://web.archive.org/web/20201021023413/https://www.iamat.org/country/morocco/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Nepal}} | |||
| | Travellers from Afghanistan, Kenya, Nigeria, Pakistan, and Papua New Guinea need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/nepal/risk/polio |title=Nepal Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=21 September 2020 |archive-url=https://web.archive.org/web/20200921102600/https://www.iamat.org/country/nepal/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Oman}} | |||
| | Travellers from polio-exporting countries need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/oman/risk/polio |title=Oman Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=15 August 2020 |archive-url=https://web.archive.org/web/20200815075731/https://www.iamat.org/country/oman/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Pakistan}} | |||
| | Travellers from ALL countries planning to stay in Pakistan for more than 4 weeks need ] proof of OPV vaccination upon arrival. Residents and all travellers staying in Pakistan longer than 4 weeks need proof of OPV vaccination when departing from Pakistan.<ref name="WHO country list"/><ref>{{Cite web |url=https://www.iamat.org/country/pakistan/risk/polio |title=Pakistan Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=21 October 2020 |archive-url=https://web.archive.org/web/20201021022622/https://www.iamat.org/country/pakistan/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Philippines}} | |||
| | Travellers from or to high-risk countries need ] proof of polio vaccination upon arrival or before departure, respectively.<ref name="WHO country list"/> Due to an ], the government recommends all others travellers to consider getting a polio vaccine or booster dose, depending on their situation.<ref>{{Cite web |url=https://www.iamat.org/country/philippines/risk/polio |title=Philippines Recommended Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=25 October 2020 |archive-url=https://web.archive.org/web/20201025045827/https://www.iamat.org/country/philippines/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Qatar}} | |||
| | Travellers from polio-exporting countries (identified by Qatar as: Afghanistan, Nigeria, Pakistan, and the Philippines) need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/qatar/risk/polio |title=Qatar Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=2 December 2020 |archive-url=https://web.archive.org/web/20201202030843/https://www.iamat.org/country/qatar/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Saint Kitts and Nevis}} | |||
| | Travellers from polio-endemic countries as identified by WHO (Afghanistan and Pakistan) need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref name="WHO country list"/><ref>{{Cite web |url=https://www.iamat.org/country/saint-kitts-and-nevis/risk/polio |title=Saint Kitts & Nevis Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=4 December 2020 |archive-url=https://web.archive.org/web/20201204075910/https://www.iamat.org/country/saint-kitts-and-nevis/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Saudi Arabia}} | |||
| | Travellers from active-transmission (including wild or vaccine-derived poliovirus) and at-risk countries, as well as all travellers from Afghanistan, Congo-Kinshasa, Mozambique, Myanmar, Niger, Nigeria, Pakistan, Papua New Guinea, Somalia, Syria, and Yemen, need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Regardless of immunisation status, all travellers from Afghanistan, Myanmar, Nigeria, Pakistan, Papua New Guinea, Somalia, Syria, and Yemen will be given an Oral Polio Vaccine dose upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/saudi-arabia/risk/polio |title=Saudi Arabia Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=27 February 2021 |archive-url=https://web.archive.org/web/20210227182449/https://www.iamat.org/country/saudi-arabia/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Seychelles}} | |||
| | Travellers from countries with polio outbreaks need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival.<ref>{{Cite web |url=https://www.iamat.org/country/seychelles/risk/polio |title=Seychelles Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=4 August 2020 |archive-url=https://web.archive.org/web/20200804144538/https://www.iamat.org/country/seychelles/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Syria}} | |||
| | Travellers from Cameroon, Equatorial Guinea, and Pakistan need ] proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. All Syria residents departing Syria to any country also need proof of vaccination.<ref>{{Cite web |url=https://www.iamat.org/country/syria/risk/polio |title=Syria Required Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=30 October 2020 |archive-url=https://web.archive.org/web/20201030073925/https://www.iamat.org/country/syria/risk/polio |url-status=live }}</ref> | |||
| |- | |||
| | {{flag|Ukraine}} | |||
| | Long-term visitors departing to states with wild or circulating vaccine-derived poliovirus transmission should present ] proof of vaccination with at least one dose of bivalent OPV or IPV (received between 4 weeks and 12 months before departure). Persons obliged to undertake urgent international travel must be immunised with a single dose of polio vaccine before their departure.<ref name="WHO country list"/> There is also risk of poliovirus transmission inside Ukraine itself, and travellers to Ukraine are recommended to be up to date with their polio vaccination before entry.<ref>{{Cite web |url=https://www.iamat.org/country/ukraine/risk/polio |title=Ukraine Recommended Vaccinations: Polio |work=iamat.org |publisher=International Association for Medical Assistance to Travellers (IAMAT) |date=23 October 2020 |access-date=2 December 2020 |archive-date=24 January 2021 |archive-url=https://web.archive.org/web/20210124000735/https://www.iamat.org/country/ukraine/risk/polio |url-status=live }}</ref> | |||
| |} | |||
| {{Clear}} | |||
| ==Society and culture== | ==Society and culture== | ||
| ===Cost=== | ===Cost=== | ||
| {{As of|2015}}, the ] supplies the inactivated vaccine to developing countries for as little as {{Currency|0.75|EURO}} (about {{USD|{{To USD|0.75|EUR}}}}) per dose in 10-dose vials.<ref>{{cite web | title = Availability and price of inactivated polio vaccine | publisher = The Global Polio Eradication Initiative | url = http://www.polioeradication.org/Mediaroom/Newsstories(old)/Newsstories2013/tabid/488/iid/354/Default.aspx#sthash.jGhJ4YUB.dpuf | archive-url = https://web.archive.org/web/20150411021753/http://www.polioeradication.org/Mediaroom/Newsstories(old)/Newsstories2013/tabid/488/iid/354/Default.aspx | archive-date = 11 April 2015 |url-status = dead}}</ref> | |||
| In the United States it costs between 25 and 50 USD for the inactivated form.<ref name=Ric2015>{{cite book|last1=Hamilton|first1=Richart|title=Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition|date=2015|publisher=Jones & Bartlett Learning|isbn=978-1-284-05756-0|page=316}}</ref> | |||
| ===Misconceptions=== | ===Misconceptions=== | ||
| A |
A misconception has been present in Pakistan that the polio vaccine contains '']'' ingredients and could cause impotence and infertility in male children, leading some parents not to have their children vaccinated. This belief is most common in the ] province and the ] region. Attacks on polio vaccination teams have also occurred, thereby hampering international efforts to eradicate polio in Pakistan and globally.<ref>{{cite news|url=http://news.bbc.co.uk/2/hi/south_asia/6299325.stm|title=Impotence fears hit polio drive|work=BBC News Online|access-date=15 December 2015|url-status = live|archive-url=https://web.archive.org/web/20151009221811/http://news.bbc.co.uk/2/hi/south_asia/6299325.stm|archive-date=9 October 2015|date=25 January 2007}}</ref><ref>{{cite web|url=http://www.dawn.com/news/1156931|title=Lab tests show polio vaccine is not 'Haram'| vauthors = Junaidi I |work=dawn.com|access-date=15 December 2015|url-status = live|archive-url=https://web.archive.org/web/20151222173149/http://www.dawn.com/news/1156931|archive-date=22 December 2015|date=14 January 2015}}</ref> | ||
| == References == | |||
| {{Reflist}} | |||
| == Further reading == | |||
| {{refbegin}} | |||
| * {{cite book | title = Immunisation against infectious disease | chapter = Polio: the green book, chapter 26 | chapter-url = https://www.gov.uk/government/publications/polio-the-green-book-chapter-26 | publisher = Public Health England | veditors = Ramsay M | url = https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book | year = 2013 | location = London | access-date = 23 December 2019 | archive-date = 12 November 2019 | archive-url = https://web.archive.org/web/20191112005859/https://www.gov.uk/government/publications/pneumococcal-the-green-book-chapter-25 | url-status = live }} | |||
| * {{cite book | publisher = U.S. ] (CDC) | title = Epidemiology and Prevention of Vaccine-Preventable Diseases | veditors = Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S | edition = 14th | location = Washington D.C. | year = 2021 | chapter = Chapter 18: Poliomyelitis | chapter-url = https://www.cdc.gov/vaccines/pubs/pinkbook/polio.html | url = https://www.cdc.gov/vaccines/pubs/pinkbook/index.html | access-date = 23 December 2019 | archive-date = 30 December 2016 | archive-url = https://web.archive.org/web/20161230001534/https://www.cdc.gov/vaccines/pubs/pinkbook/index.html | url-status = live }} | |||
| * {{cite book | vauthors = Routh JA, Oberste MS, Patel M | chapter = Chapter 12: Poliomyelitis | chapter-url = https://www.cdc.gov/vaccines/pubs/surv-manual/chpt12-polio.html | veditors = Roush SW, Baldy LM, Hall MH | title = Manual for the surveillance of vaccine-preventable diseases | publisher = U.S. ] (CDC) | location = Atlanta, Georgia | url = https://www.cdc.gov/vaccines/pubs/surv-manual/ | year = 2018 | access-date = 23 December 2019 | archive-date = 1 August 2020 | archive-url = https://web.archive.org/web/20200801192220/https://www.cdc.gov/vaccines/pubs/surv-manual/ | url-status = live }} | |||
| {{refend}} | |||
| == External links == | |||
| ==References== | |||
| * {{cite web | title=Polio Vaccine Information Statement | website=] (CDC) | date=August 2021 | url=https://www.cdc.gov/vaccines/hcp/vis/vis-statements/ipv.html }} | |||
| {{Reflist|30em}} | |||
| * History of Vaccines, a project of the ] | |||
| * – 'People and Discoveries: Salk Produces Polio Vaccine 1952', ] (PBS) | |||
| * {{cite web | title=IPOL – Poliovirus Vaccine Inactivated (Monkey Kidney Cell) | website=U.S. ] (FDA) | date=11 December 2019 | url=https://www.fda.gov/vaccines-blood-biologics/vaccines/ipol-poliovirus-vaccine-inactivated-monkey-kidney-cell | id=STN: 103930 }} | |||
| * {{MeshName|Poliovirus Vaccines}} | |||
| ==External links== | |||
| * final project to eliminate polio by 2018. | |||
| * History of Vaccines, a project of the ] | |||
| * — 'People and Discoveries: Salk Produces Polio Vaccine 1952', ] | |||
| * , Smithsonian Magazine, April 2005 | |||
| * , Dream 2047 Magazine, April 2004 | |||
| {{Vaccines}} | {{Vaccines}} | ||
| {{good article}} | |||
| {{Portal bar|Medicine|Viruses}} | {{Portal bar|Medicine|Viruses}} | ||
| {{Authority control}} | |||
| {{DEFAULTSORT:Polio Vaccine}} | {{DEFAULTSORT:Polio Vaccine}} | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
Latest revision as of 02:30, 20 December 2024
Vaccine to prevent poliomyelitisPharmaceutical compound
 | |
| Vaccine description | |
|---|---|
| Target | Poliomyelitis |
| Vaccine type | IPV: inactivated OPV: attenuated nOPV2: attenuated, genetically stabilised |
| Clinical data | |
| Trade names | Ipol, Poliovax, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601177 |
| License data | |
| Pregnancy category |
|
| Routes of administration | IPV: parenteral OPV: oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| (what is this?) (verify) | |
Polio vaccines are vaccines used to prevent poliomyelitis (polio). Two types are used: an inactivated poliovirus given by injection (IPV) and a weakened poliovirus given by mouth (OPV). The World Health Organization (WHO) recommends all children be fully vaccinated against polio. The two vaccines have eliminated polio from most of the world, and reduced the number of cases reported each year from an estimated 350,000 in 1988 to 33 in 2018.
The inactivated polio vaccines are very safe. Mild redness or pain may occur at the site of injection. Oral polio vaccines cause about three cases of vaccine-associated paralytic poliomyelitis per million doses given. This compares with 5,000 cases per million who are paralysed following a polio infection. Both types of vaccine are generally safe to give during pregnancy and in those who have HIV/AIDS but are otherwise well. However, the emergence of circulating vaccine-derived poliovirus (cVDPV), a form of the vaccine virus that has reverted to causing poliomyelitis, has led to the development of novel oral polio vaccine type 2 (nOPV2) which aims to make the vaccine safer and thus stop further outbreaks of cVDPV.
The first successful demonstration of a polio vaccine was by Hilary Koprowski in 1950, with a live attenuated virus which people drank. The vaccine was not approved for use in the United States, but was used successfully elsewhere. The success of an inactivated (killed) polio vaccine, developed by Jonas Salk, was announced in 1955. Another attenuated live oral polio vaccine was developed by Albert Sabin and came into commercial use in 1961.
Polio vaccine is on the World Health Organization's List of Essential Medicines.
Medical uses

Interruption of person-to-person transmission of the virus by vaccination is important in global polio eradication, since no long-term carrier state exists for poliovirus in individuals with normal immune function, polio viruses have no non-primate reservoir in nature, and survival of the virus in the environment for an extended period appears to be remote. There are two types of vaccine: inactivated polio vaccine (IPV) and oral polio vaccine (OPV).
Inactivated
When the IPV (injection) is used, 90% or more of individuals develop protective antibodies to all three serotypes of polio virus after two doses of inactivated polio vaccine (IPV), and at least 99% are immune to poliovirus following three doses. The duration of immunity induced by IPV is not known with certainty, although a complete series is thought to protect for many years. IPV replaced the oral vaccine in many developed countries in the 1990s mainly due to the (small) risk of vaccine-derived polio in the oral vaccine.
Attenuated
Oral polio vaccines were easier to administer than IPV, as they eliminated the need for sterile syringes and therefore were more suitable for mass vaccination campaigns. OPV also provided longer-lasting immunity than the Salk vaccine, as it provides both humoral immunity and cell-mediated immunity.
One dose of trivalent OPV produces immunity to all three poliovirus serotypes in roughly 50% of recipients. Three doses of live-attenuated OPV produce protective antibodies to all three poliovirus types in more than 95% of recipients. As with other live-virus vaccines, immunity initiated by OPV is probably lifelong. OPV produces excellent immunity in the intestine, the primary site of wild poliovirus entry, which helps prevent infection with wild virus in areas where the virus is endemic. The oral administration does not require special medical equipment or extensive training. Attenuated poliovirus derived from the oral polio vaccine is excreted for a few days after vaccination, potentially infecting and thus indirectly inducing immunity in unvaccinated individuals, and thus amplifying the effects of the doses delivered. Taken together, these advantages have made it the favored vaccine of many countries, and it has long been preferred by the global eradication initiative.
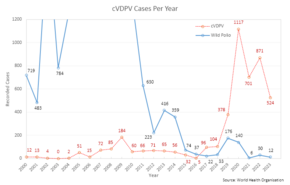
The primary disadvantage of OPV derives from its inherent nature. As an attenuated but active virus, it can induce vaccine-associated paralytic poliomyelitis (VAPP) in approximately one individual per every 2.7 million doses administered. The live virus can circulate in under-vaccinated populations (termed either variant poliovirus or circulating vaccine-derived poliovirus, cVDPV) and over time can revert to a neurovirulent form causing paralytic polio. This genetic reversal of the pathogen to a virulent form takes a considerable time and does not affect the person who was originally vaccinated. With wild polio cases at record lows, 2017 was the first year where more cases of cVDPV were recorded than the wild poliovirus.
Until recent times, a trivalent OPV containing all three virus strains was used, and had nearly eradicated polio infection worldwide. With the complete eradication of wild poliovirus type 2 this was phased out in 2016 and replaced with bivalent vaccine containing just types 1 and 3, supplemented with monovalent type 2 OPV in regions where cVDPV type 2 was known to circulate. The switch to the bivalent vaccine and associated missing immunity against type 2 strains, among other factors, led to outbreaks of circulating vaccine-derived poliovirus type 2 (cVDPV2), which increased from 2 cases in 2016 to 1037 cases in 2020.
A novel OPV2 vaccine (nOPV2) which has been genetically modified to reduce the likelihood of disease-causing activating mutations was granted emergency licencing in 2021, and subsequently full licensure in December 2023. This has greater genetic stability than the traditional oral vaccine and is less likely to revert to a virulent form. Genetically stabilised vaccines targeting poliovirus types 1 and 3 are in development, with the intention that these will eventually completely replace the Sabin vaccines.
Schedule
In countries with endemic polio or where the risk of imported cases is high, the WHO recommends OPV vaccine at birth followed by a primary series of three OPV doses and at least one IPV dose starting at 6 weeks of age, with a minimum of 4 weeks between OPV doses. In countries with >90% immunization coverage and low risk of importation, the WHO recommends one or two IPV doses starting at 2 months of age followed by at least two OPV doses, with the doses separated by 4–8 weeks depending on the risk of exposure. In countries with the highest levels of coverage and the lowest risks of importation and transmission, the WHO recommends a primary series of three IPV injections, with a booster dose after an interval of six months or more if the first dose was administered before 2 months of age.
Side effects
The inactivated polio vaccines are very safe. Mild redness or pain may occur at the site of injection. They are generally safe to be given to pregnant women and those who have HIV/AIDS but are otherwise well.
Allergic reaction to the vaccine
Inactivated polio vaccine can cause an allergic reaction in a few people since the vaccine contains trace amounts of antibiotics, streptomycin, polymyxin B, and neomycin. It should not be given to anyone who has an allergic reaction to these medicines. Signs and symptoms of an allergic reaction, which usually appear within minutes or a few hours after receiving the injected vaccine, include breathing difficulties, weakness, hoarseness or wheezing, heart rate fluctuations, skin rash, and dizziness.
Vaccine-associated paralytic polio
A potential adverse effect of the Sabin OPV is caused by its known potential to recombine to a form that causes neurological infection and paralysis. The Sabin OPV results in vaccine-associated paralytic poliomyelitis (VAPP) in approximately one individual per every 2.7 million doses administered, with symptoms identical to wild polio. Due to its improved genetic stability, the novel OPV (nOPV) has a reduced risk of this occurring.
Contamination concerns
Further information: Vaccine contamination with SV40In 1960, the rhesus monkey kidney cells used to prepare the poliovirus vaccines were determined to be infected with the simian virus-40 (SV40), which was also discovered in 1960 and is a naturally occurring virus that infects monkeys. In 1961, SV40 was found to cause tumors in rodents. More recently, the virus was found in certain forms of cancer in humans, for instance brain and bone tumors, pleural and peritoneal mesothelioma, and some types of non-Hodgkin lymphoma. However, SV40 has not been determined to cause these cancers.
SV40 was found to be present in stocks of the injected form of the IPV in use between 1955 and 1963. It is not found in the OPV form. Over 98 million Americans received one or more doses of polio vaccine between 1955 and 1963 when a proportion of vaccine was contaminated with SV40; an estimated 10–30 million Americans may have received a dose of vaccine contaminated with SV40. Later analysis suggested that vaccines produced by the former Soviet bloc countries until 1980, and used in the USSR, China, Japan, and several African countries, may have been contaminated, meaning hundreds of millions more may have been exposed to SV40.
In 1998, the National Cancer Institute undertook a large study, using cancer case information from the institute's SEER database. The published findings from the study revealed no increased incidence of cancer in persons who may have received vaccine containing SV40. Another large study in Sweden examined cancer rates of 700,000 individuals who had received potentially contaminated polio vaccine as late as 1957; the study again revealed no increased cancer incidence between persons who received polio vaccines containing SV40 and those who did not. The question of whether SV40 causes cancer in humans remains controversial, however, and the development of improved assays for detection of SV40 in human tissues will be needed to resolve the controversy.

During the race to develop an oral polio vaccine, several large-scale human trials were undertaken. By 1958, the National Institutes of Health had determined that OPV produced using the Sabin strains was the safest. Between 1957 and 1960, however, Hilary Koprowski continued to administer his vaccine around the world. In Africa, the vaccines were administered to roughly one million people in the Belgian territories (now the Democratic Republic of the Congo, Rwanda, and Burundi). The results of these human trials have been controversial, and unfounded accusations in the 1990s arose that the vaccine had created the conditions necessary for transmission of simian immunodeficiency virus from chimpanzees to humans, causing HIV/AIDS. These hypotheses, however, have been conclusively refuted. By 2004, cases of poliomyelitis in Africa had been reduced to just a small number of isolated regions in the western portion of the continent, with sporadic cases elsewhere. Recent local opposition to vaccination campaigns have evolved due to lack of adequate information, often relating to fears that the vaccine might induce sterility. The disease has since resurged in Nigeria and in several other African nations without necessary information, which epidemiologists believe is due to refusals by certain local populations to allow their children to receive the polio vaccine.
Manufacture
Inactivated
The Salk vaccine, IPV, is based on three wild, virulent reference strains, Mahoney (type 1 poliovirus), MEF-1 (type 2 poliovirus), and Saukett (type 3 poliovirus), grown in a type of monkey kidney tissue culture (Vero cell line), which are then inactivated with formalin. The injected Salk vaccine confers IgG-mediated immunity in the bloodstream, which prevents polio infection from progressing to viremia and protects the motor neurons, thus eliminating the risk of bulbar polio and post-polio syndrome.
In the United States, the vaccine is administered along with the tetanus, diphtheria, and acellular pertussis vaccines (DTaP) and a pediatric dose of hepatitis B vaccine. In the UK, IPV is combined with tetanus, diphtheria, pertussis, and Haemophilus influenzae type b vaccines.
Attenuated


OPV is an attenuated vaccine, produced by the passage of the virus through nonhuman cells at a subphysiological temperature, which produces spontaneous mutations in the viral genome. Oral polio vaccines were developed by several groups, one of which was led by Albert Sabin. Other groups, led by Hilary Koprowski and H.R. Cox, developed their attenuated vaccine strains. In 1958, the National Institutes of Health created a special committee on live polio vaccines. The various vaccines were carefully evaluated for their ability to induce immunity to polio while retaining a low incidence of neuropathogenicity in monkeys. Large-scale clinical trials performed in the Soviet Union in the late 1950s to early 1960s by Mikhail Chumakov and his colleagues demonstrated the safety and high efficacy of the vaccine. Based on these results, the Sabin strains were chosen for worldwide distribution. Fifty-seven nucleotide substitutions distinguish the attenuated Sabin 1 strain from its virulent parent (the Mahoney serotype), two nucleotide substitutions attenuate the Sabin 2 strain, and 10 substitutions are involved in attenuating the Sabin 3 strain. The primary attenuating factor common to all three Sabin vaccines is a mutation located in the virus's internal ribosome entry site, which alters stem-loop structures and reduces the ability of poliovirus to translate its RNA template within the host cell. The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of infection and replication, but is unable to replicate efficiently within nervous system tissue. In 1961, type 1 and 2 monovalent oral poliovirus vaccine (MOPV) was licensed, and in 1962, type 3 MOPV was licensed. In 1963, trivalent OPV (TOPV) was licensed, and became the vaccine of choice in the United States and most other countries of the world, largely replacing the inactivated polio vaccine. A second wave of mass immunizations led to a further dramatic decline in the number of polio cases. Between 1962 and 1965, about 100 million Americans (roughly 56% of the population at that time) received the Sabin vaccine. The result was a substantial reduction in the number of poliomyelitis cases, even from the much-reduced levels following the introduction of the Salk vaccine.
OPV is usually provided in vials containing 10–20 doses of vaccine. A single dose of oral polio vaccine (usually two drops) contains 1,000,000 infectious units of Sabin 1 (effective against PV1), 100,000 infectious units of the Sabin 2 strain, and 600,000 infectious units of Sabin 3. The vaccine contains small traces of antibiotics—neomycin and streptomycin—but does not contain preservatives.
History
See also: Cold War tensions and the polio vaccineIn a generic sense, vaccination works by priming the immune system with an 'immunogen'. Stimulating immune response, by use of an infectious agent, is known as immunization. The development of immunity to polio efficiently blocks person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community.
The development of two polio vaccines led to the first modern mass inoculations. The last cases of paralytic poliomyelitis caused by endemic transmission of wild virus in the United States occurred in 1979, with an outbreak among the Amish in several Midwest states.
1930s
In the 1930s, poliovirus was perceived as especially terrifying, as little was known of how the disease was transmitted or how it could be prevented. This virus was also notable for primarily impacting affluent children, making it a prime target for vaccine development, despite its relatively low mortality and morbidity. Despite this, the community of researchers in the field thus far had largely observed an informal moratorium on any vaccine development as it was perceived to present too high a risk for too little likelihood of success.
This shifted in the early 1930s when American groups took up the challenge: Maurice Brodie led a team from the public health laboratory of the city of New York and John A. Kolmer collaborated with the Research Institute of Cutaneous Medicine in Philadelphia. The rivalry between these two researchers lent itself to a race-like mentality which, combined with a lack of oversight of medical studies, was reflected in the methodology and outcomes of each of these early vaccine development ventures.
Kolmer's live vaccine
Kolmer began his vaccine development project in 1932 and ultimately focused on producing an attenuated or live virus vaccine. Inspired by the success of vaccines for rabies and yellow fever, he hoped to use a similar process to denature the polio virus. In order to go about attenuating his polio vaccine, he repeatedly passed the virus through monkeys. Using methods of production that were later described as "hair-raisingly amateurish, the therapeutic equivalent of bath-tub gin," Kolmer ground the spinal cords of his infected monkeys and soaked them in a salt solution. He then filtered the solution through mesh, treated it with ricinolate, and refrigerated the product for 14 days to ultimately create what would later be prominently critiqued as a "veritable witches brew".
In keeping with the norms of the time, Kolmer completed a relatively small animal trial with 42 monkeys before proceeding to self experimentation in 1934. He tested his vaccine upon himself, his two children, and his assistant. He gave his vaccine to just 23 more children before declaring it safe and sending it out to doctors and health departments for a larger test of efficacy. By April 1935, he was able to report having tested the vaccine on 100 children without ill effect. Kolmer's first formal presentation of results would not come about until November 1935 where he presented the results of 446 children and adults he had vaccinated with his attenuated vaccine. He also reported that together the Research Institute of Cutaneous Medicine and the Merrell Company of Cincinnati (the manufacturer who held the patent for his ricinoleating process) had distributed 12,000 doses of vaccine to some 700 physicians across the United States and Canada. Kolmer did not describe any monitoring of this experimental vaccination program nor did he provide these physicians with instructions in how to administer the vaccine or how to report side effects. Kolmer dedicated the bulk of his publications thereafter to explaining what he believed to be the cause of the 10+ reported cases of paralytic polio following vaccination, in many cases in towns where no polio outbreak had occurred. Six of these cases had been fatal. Kolmer had no control group but asserted that many more children would have gotten sick.
Brodie's inactivated vaccine
At nearly the same time as Kolmer's project, Maurice Brodie had joined immunologist William H. Park at the New York City Health Department where they worked together on poliovirus. With the aid of grant funding from the President's Birthday Ball Commission (a predecessor to what would become the March of Dimes), Brodie was able to pursue the development of an inactivated or "killed virus" vaccine. Brodie's process also began by grinding the spinal cords of infectious monkeys and then treating the cords with various germicides, ultimately finding a solution of formaldehyde to be the most effective. By 1 June 1934, Brodie was able to publish his first scholarly article describing his successful induction of immunity in three monkeys with inactivated poliovirus. Through continued study on an additional 26 monkeys, Brodie ultimately concluded that administration of live virus vaccine tended to result in humoral immunity while administration of killed virus vaccine tended to result in tissue immunity.
Soon after, following a similar protocol to Kolmer, Brodie proceeded with self-experimentation upon himself and his co-workers at the NYC Health Department laboratory. Brodie's progress was eagerly covered by popular press as the public hoped for a successful vaccine to become available. Such reporting did not make mention of the 12 children in a New York City Asylum who were subjected to early safety trials. As none of the subjects experienced ill effects, Park, described by contemporaries as "never one to let grass grow under his feet," declared the vaccine safe. When a severe polio outbreak overwhelmed Kern County, California it became the first trial site for the new vaccine on very short notice. Between November 1934 - May 1935, over 1,500 doses of the vaccine were administered in Kern County. While initial results were very promising, insufficient staffing and poor protocol design left Brodie open to criticism when he published the California results in August 1935. Through private physicians, Brodie also conducted a broader field study, including 9,000 children who received the vaccine and 4,500 age- and location-matched controls who did not receive a vaccine. Again, the results were promising. Of those who received the vaccine, only a few went on to develop polio. Most had been exposed before vaccination and none had received the full series of vaccine doses being studied. Additionally, a polio epidemic in Raleigh, North Carolina provided an opportunity for the U.S. Public Health Service to conduct a highly structured trial of the Brodie vaccine using funding from the Birthday Ball Commission.
Academic reception
While their work was ongoing, the larger community of bacteriologists began to raise concerns regarding the safety and efficacy of the new poliovirus vaccines. At this time there was very little oversight of medical studies and the ethical treatment of study participants largely relied upon moral pressure from peer academic scientists. Brodie's inactivated vaccines faced scrutiny from many who felt killed virus vaccines could not be efficacious. While researchers were able to replicate the tissue immunity he had produced in his animal trials, the prevailing wisdom was that humoral immunity was essential for an efficacious vaccine. Kolmer directly questioned the killed virus approach in scholarly journals. Kolmer's studies however had raised even more concern with increasing reports of children becoming paralysed following vaccination with his live virus vaccine and notably, with paralysis beginning at the arm rather than the foot in many cases. Both Kolmer and Brodie were called to present their research at the Annual Meeting of the American Public Health Association in Milwaukee WI in October 1935. Additionally, Thomas M. Rivers was asked to discuss each of the presented papers as a prominent critic of the vaccine development effort. This resulted in the APHA arranging a Symposium on Poliomyelitis to be delivered at the Annual Meeting of their Southern Branch the following month. It was during the discussion at this meeting that James Leake of the U.S. Public Health Service stood to immediately present clinical evidence that the Kolmer vaccine had caused several deaths and then allegedly accused Kolmer of being a murderer. As Rivers recalled in his oral history, "All hell broke loose, and it seemed as if everybody was trying to talk at the same time...Jimmy Leake used the strongest language that I have ever heard used at a scientific meeting." In response to the attacks from all sides, Brodie was reported to have stood up and stated, "It looks as though, according to Dr. Rivers, my vaccine is no good, and, according to Dr. Leake, Dr Kolmer's is dangerous." Kolmer simply responded by stating, "Gentlemen, this is one time I wish the floor would open up and swallow me." Ultimately, Kolmer's live vaccine was undoubtedly shown to be dangerous and had already been withdrawn in September 1935 before the Milwaukee meeting. While the consensus of the symposium was largely skeptical of the efficacy of Brodie's vaccine, its safety was not in question and the recommendation was for a much larger well-controlled trial. However, when three children became ill with paralytic polio following a dose of the vaccine, the directors of the Warm Springs Foundation in Georgia (acting as the primary funders for the project) requested it be withdrawn in December 1935. Following its withdrawal, the previously observed moratorium on human poliomyelitis vaccine development resumed and there would not be another attempt for nearly 20 years.
While Brodie had arguably made the most progress in the pursuit of a poliovirus vaccine, he suffered the most significant career repercussions due to his status as a less widely known researcher. Modern researchers recognize that Brodie may well have developed an effective polio vaccine, however, the basic science and technology of the time were insufficient to understand and utilize this breakthrough. Brodie's work using formalin-inactivated virus would later become the basis for the Salk vaccine, but he would not live to see this success. Brodie was fired from his position within three months of the symposium's publication. While he was able to find another laboratory position, he died of a heart attack only three years later at age 36. By contrast, Park, who was believed in the community to be reaching senility at this point in his older age, was able to retire from his position with honors before he died in 1939. Kolmer, already an established and well-respected researcher, returned to Temple University as a professor of medicine. Kolmer had a very productive career, receiving multiple awards, and publishing countless papers, articles, and textbooks up until his retirement in 1957.
1948
A breakthrough came in 1948 when a research group headed by John Enders at the Children's Hospital Boston successfully cultivated the poliovirus in human tissue in the laboratory. This group had recently successfully grown mumps in cell culture. In March 1948, Thomas H. Weller was attempting to grow varicella virus in embryonic lung tissue. He had inoculated the planned number of tubes when he noticed that there were a few unused tubes. He retrieved a sample of mouse brain infected with poliovirus and added it to the remaining test tubes, on the off chance that the virus might grow. The varicella cultures failed to grow, but the polio cultures were successful. This development greatly facilitated vaccine research and ultimately allowed for the development of vaccines against polio. Enders and his colleagues, Thomas H. Weller and Frederick C. Robbins, were recognized in 1954 for their efforts with a Nobel Prize in Physiology or Medicine. Other important advances that led to the development of polio vaccines were: the identification of three poliovirus serotypes (Poliovirus type 1 – PV1, or Mahoney; PV2, Lansing; and PV3, Leon); the finding that before paralysis, the virus must be present in the blood; and the demonstration that administration of antibodies in the form of gamma globulin protects against paralytic polio.
1950–1955
See also: Announcement of Polio vaccine success
During the early 1950s, polio rates in the U.S. were above 25,000 annually; in 1952 and 1953, the U.S. experienced an outbreak of 58,000 and 35,000 polio cases, respectively, up from a typical number of some 20,000 a year, with deaths in those years numbering 3,200 and 1,400. Amid this U.S. polio epidemic, millions of dollars were invested in finding and marketing a polio vaccine by commercial interests, including Lederle Laboratories in New York under the direction of H. R. Cox. Also working at Lederle was Polish-born virologist and immunologist Hilary Koprowski of the Wistar Institute in Philadelphia, who tested the first successful polio vaccine, in 1950. His vaccine, however, being a live attenuated virus taken orally, was still in the research stage and would not be ready for use until five years after Jonas Salk's polio vaccine (a dead-virus injectable vaccine) had reached the market. Koprowski's attenuated vaccine was prepared by successive passages through the brains of Swiss albino mice. By the seventh passage, the vaccine strains could no longer infect nervous tissue or cause paralysis. After one to three further passages on rats, the vaccine was deemed safe for human use. On 27 February 1950, Koprowski's live, attenuated vaccine was tested for the first time on an 8-year-old boy living at Letchworth Village, an institution for physically and mentally disabled people located in New York. After the child had no side effects, Koprowski enlarged his experiment to include 19 other children.
Jonas Salk


The first effective polio vaccine was developed in 1952 by Jonas Salk and a team at the University of Pittsburgh that included Julius Youngner, Byron Bennett, L. James Lewis, and Lorraine Friedman, which required years of subsequent testing. Salk went on CBS radio to report a successful test on a small group of adults and children on 26 March 1953; two days later, the results were published in JAMA. Leone N. Farrell invented a key laboratory technique that enabled the mass production of the vaccine by a team she led in Toronto. Beginning 23 February 1954, the vaccine was tested at Arsenal Elementary School and the Watson Home for Children in Pittsburgh, Pennsylvania.
Salk's vaccine was then used in a test called the Francis Field Trial, led by Thomas Francis, the largest medical experiment in history at that time. The test began with about 4,000 children at Franklin Sherman Elementary School in McLean, Virginia, and eventually involved 1.8 million children, in 44 states from Maine to California. By the conclusion of the study, roughly 440,000 received one or more injections of the vaccine, about 210,000 children received a placebo, consisting of harmless culture media, and 1.2 million children received no vaccination and served as a control group, who would then be observed to see if any contracted polio.
The results of the field trial were announced on 12 April 1955 (the tenth anniversary of the death of President Franklin D. Roosevelt, whose paralytic illness was generally believed to have been caused by polio). The Salk vaccine had been 60–70% effective against PV1 (poliovirus type 1), over 90% effective against PV2 and PV3, and 94% effective against the development of bulbar polio. Soon after Salk's vaccine was licensed in 1955, children's vaccination campaigns were launched. In the U.S., following a mass immunization campaign promoted by the March of Dimes, the annual number of polio cases fell from 35,000 in 1953 to 5,600 by 1957. By 1961 only 161 cases were recorded in the United States.
A week before the announcement of the Francis Field Trial results in April 1955, Pierre Lépine at the Pasteur Institute in Paris had also announced an effective polio vaccine.
Safety incidents
In April 1955, soon after mass polio vaccination began in the US, the Surgeon General began to receive reports of patients who contracted paralytic polio about a week after being vaccinated with the Salk polio vaccine from the Cutter pharmaceutical company, with the paralysis starting in the limb the vaccine was injected into. The Cutter vaccine had been used in vaccinating 409,000 children in the western and midwestern United States. Later investigations showed that the Cutter vaccine had caused 260 cases of polio, killing 11. In response, the Surgeon General pulled all polio vaccines made by Cutter Laboratories from the market, but not before 260 cases of paralytic illness had occurred. Eli Lilly, Parke-Davis, Pitman-Moore, and Wyeth polio vaccines were also reported to have paralyzed numerous children. It was soon discovered that some lots of Salk polio vaccine made by Cutter, Wyeth, and the other labs had not been properly inactivated, allowing live poliovirus into more than 100,000 doses of vaccine. In May 1955, the National Institutes of Health and Public Health Services established a Technical Committee on Poliomyelitis Vaccine to test and review all polio vaccine lots and advise the Public Health Service as to which lots should be released for public use. These incidents reduced public confidence in the polio vaccine, leading to a drop in vaccination rates.
1961

At the same time that Salk was testing his vaccine, both Albert Sabin and Hilary Koprowski continued working on developing a vaccine using live virus. During a meeting in Stockholm to discuss polio vaccines in November 1955, Sabin presented results obtained on a group of 80 volunteers, while Koprowski read a paper detailing the findings of a trial enrolling 150 people. Sabin and Koprowski both eventually succeeded in developing vaccines. Because of the commitment to the Salk vaccine in America, Sabin and Koprowski both did their testing outside the United States, Sabin in Mexico and the Soviet Union, Koprowski in the Congo and Poland. In 1957, Sabin developed a trivalent vaccine containing attenuated strains of all three types of poliovirus. In 1959, ten million children in the Soviet Union received the Sabin oral vaccine. For this work, Sabin was given the medal of the Order of Friendship of Peoples, described as the Soviet Union's highest civilian honor. Sabin's oral vaccine using live virus came into commercial use in 1961.
Once Sabin's oral vaccine became widely available, it supplanted Salk's injected vaccine, which had been tarnished in the public's opinion by the Cutter incident of 1955, in which Salk vaccines improperly prepared by one company resulted in several children dying or becoming paralyzed.
1987
An enhanced-potency IPV was licensed in the United States in November 1987, and is currently the vaccine of choice there. The first dose of the polio vaccine is given shortly after birth, usually between 1 and 2 months of age, and a second dose is given at 4 months of age. The timing of the third dose depends on the vaccine formulation but should be given between 6 and 18 months of age. A booster vaccination is given at 4 to 6 years of age, for a total of four doses at or before school entry. In some countries, a fifth vaccination is given during adolescence. Routine vaccination of adults (18 years of age and older) in developed countries is neither necessary nor recommended because most adults are already immune and have a very small risk of exposure to wild poliovirus in their home countries. In 2002, a pentavalent (five-component) combination vaccine (called Pediarix) containing IPV was approved for use in the United States.
1988

A global effort to eradicate polio, led by the World Health Organization (WHO), UNICEF, and the Rotary Foundation, began in 1988, and has relied largely on the oral polio vaccine developed by Albert Sabin and Mikhail Chumakov (Sabin-Chumakov vaccine).
After 1990
Polio was eliminated in the Americas by 1994. The disease was officially eliminated in 36 Western Pacific countries, including China and Australia, in 2000. Europe was declared polio-free in 2002. Since January 2011, no cases of the disease have been reported in India, hence in February 2012, the country was taken off the WHO list of polio-endemic countries. In March 2014, India was declared a polio-free country.
Although poliovirus transmission has been interrupted in much of the world, transmission of wild poliovirus does continue and creates an ongoing risk for the importation of wild poliovirus into previously polio-free regions. If importations of poliovirus occur, outbreaks of poliomyelitis may develop, especially in areas with low vaccination coverage and poor sanitation. As a result, high levels of vaccination coverage must be maintained. In November 2013, the WHO announced a polio outbreak in Syria. In response, the Armenian government put out a notice asking Syrian Armenians under age 15 to get the polio vaccine. As of 2014, polio virus had spread to 10 countries, mainly in Africa, Asia, and the Middle East, with Pakistan, Syria, and Cameroon advising vaccinations to outbound travellers.
Polio vaccination programs have been resisted by some people in Pakistan, Afghanistan, and Nigeria - the three countries as of 2017 with remaining polio cases. Almost all Muslim religious and political leaders have endorsed the vaccine, but a fringe minority believes that the vaccines are secretly being used for the sterilisation of Muslims. The fact that the CIA organized a fake vaccination program in 2011 to help find Osama bin Laden is an additional cause of distrust. In 2015, the WHO announced a deal with the Taliban to encourage them to distribute the vaccine in areas they control. However, the Pakistani Taliban was not supportive. On 11 September 2016, two unidentified gunmen associated with the Pakistani Taliban, Jamaat-ul-Ahrar, shot Zakaullah Khan, a doctor who was administering polio vaccines in Pakistan. The leader of the Jamaat-ul-Ahrar claimed responsibility for the shooting and stated that the group would continue this type of attack. Such resistance to and skepticism of vaccinations has consequently slowed down the polio eradication process within the two remaining endemic countries.
Travel requirements
Travellers who wish to enter or leave certain countries must be vaccinated against polio, usually at most 12 months and at least 4 weeks before crossing the border, and be able to present a vaccination record/certificate at the border checks. Most requirements apply only to travel to or from so-called 'polio-endemic', 'polio-affected', 'polio-exporting', 'polio-transmission', or 'high-risk' countries. As of August 2020, Afghanistan and Pakistan are the only polio-endemic countries in the world (where wild polio has not yet been eradicated). Several countries have additional precautionary polio vaccination travel requirements, for example to and from 'key at-risk countries', which as of December 2020 include China, Indonesia, Mozambique, Myanmar, and Papua New Guinea.
| Polio vaccination requirements for international travel | |
|---|---|
| Country | Details |
| Travellers from polio-endemic countries (Pakistan) need Carte Jaune proof of polio vaccination (received between 4 weeks and 12 months before departure) upon arrival. Residents and all travellers staying in Afghanistan longer than 4 weeks need proof of polio vaccination (received between 4 weeks and 12 months before departure) when departing from Afghanistan. | |
| Travellers from Afghanistan and Pakistan need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Belize residents travelling to countries with confirmed polio cases also need proof of vaccination. | |
| Travellers from polio-exporting countries need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from Afghanistan, Angola, Benin, Cameroon, the Central African Republic, China, Congo-Kinshasa, Ethiopia, Ghana, Indonesia, Kenya, Mozambique, Myanmar, Niger, Nigeria, Pakistan, Papua New Guinea, Philippines, and Somalia need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from at-risk countries need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Travellers without proof are offered OPV vaccination upon arrival. | |
| Travellers from Afghanistan, Congo-Kinshasa, Ethiopia, Kenya, Nigeria, Pakistan, Somalia, and Syria need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from Afghanistan, Pakistan, and Nigeria need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Travellers without proof will be vaccinated upon arrival. | |
| Travellers aged 15+ from Afghanistan and Pakistan need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival; children under age 15 must have received three doses of polio vaccine before travel. Travellers without proof will be vaccinated upon arrival. Travellers departing from Iraq to Afghanistan and Pakistan must also provide proof of vaccination upon departure. | |
| Travellers from Afghanistan and Pakistan need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from and to polio-affected countries need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from Afghanistan and Pakistan need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from and to polio-exporting countries, as well as Hajj and Umrah pilgrims, need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from polio-affected countries need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from Afghanistan, Kenya, Nigeria, Pakistan, and Papua New Guinea need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from polio-exporting countries need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from ALL countries planning to stay in Pakistan for more than 4 weeks need Carte Jaune proof of OPV vaccination upon arrival. Residents and all travellers staying in Pakistan longer than 4 weeks need proof of OPV vaccination when departing from Pakistan. | |
| Travellers from or to high-risk countries need Carte Jaune proof of polio vaccination upon arrival or before departure, respectively. Due to an ongoing local VDPV2 outbreak, the government recommends all others travellers to consider getting a polio vaccine or booster dose, depending on their situation. | |
| Travellers from polio-exporting countries (identified by Qatar as: Afghanistan, Nigeria, Pakistan, and the Philippines) need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from polio-endemic countries as identified by WHO (Afghanistan and Pakistan) need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from active-transmission (including wild or vaccine-derived poliovirus) and at-risk countries, as well as all travellers from Afghanistan, Congo-Kinshasa, Mozambique, Myanmar, Niger, Nigeria, Pakistan, Papua New Guinea, Somalia, Syria, and Yemen, need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. Regardless of immunisation status, all travellers from Afghanistan, Myanmar, Nigeria, Pakistan, Papua New Guinea, Somalia, Syria, and Yemen will be given an Oral Polio Vaccine dose upon arrival. | |
| Travellers from countries with polio outbreaks need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. | |
| Travellers from Cameroon, Equatorial Guinea, and Pakistan need Carte Jaune proof of OPV or IPV vaccination (received between 4 weeks and 12 months before departure) upon arrival. All Syria residents departing Syria to any country also need proof of vaccination. | |
| Long-term visitors departing to states with wild or circulating vaccine-derived poliovirus transmission should present Carte Jaune proof of vaccination with at least one dose of bivalent OPV or IPV (received between 4 weeks and 12 months before departure). Persons obliged to undertake urgent international travel must be immunised with a single dose of polio vaccine before their departure. There is also risk of poliovirus transmission inside Ukraine itself, and travellers to Ukraine are recommended to be up to date with their polio vaccination before entry. | |
Society and culture
Cost
As of 2015, the Global Alliance for Vaccines and Immunization supplies the inactivated vaccine to developing countries for as little as €0.75 (about US$0.89) per dose in 10-dose vials.
Misconceptions
A misconception has been present in Pakistan that the polio vaccine contains haram ingredients and could cause impotence and infertility in male children, leading some parents not to have their children vaccinated. This belief is most common in the Khyber Pakhtunkhwa province and the FATA region. Attacks on polio vaccination teams have also occurred, thereby hampering international efforts to eradicate polio in Pakistan and globally.
References
- Use During Pregnancy and Breastfeeding
- ^ World Health Organization (2016). "Polio vaccines: WHO position paper – March, 2016". Weekly Epidemiological Record. 91 (12): 145–68. hdl:10665/254399. PMID 27039410.
- World Health Organization (2022). "Polio vaccines: WHO position paper – June 2022". Weekly Epidemiological Record. 97 (25): 277–300. hdl:10665/357168.
- Aylward RB (2006). "Eradicating polio: today's challenges and tomorrow's legacy". Annals of Tropical Medicine and Parasitology. 100 (5–6): 401–413. doi:10.1179/136485906X97354. PMID 16899145. S2CID 25327986.
- Schonberger LB, Kaplan J, Kim-Farley R, Moore M, Eddins DL, Hatch M (1984). "Control of paralytic poliomyelitis in the United States". Reviews of Infectious Diseases. 6 (Suppl 2): S424 – S426. doi:10.1093/clinids/6.Supplement_2.S424. PMID 6740085.
- "Global Wild Poliovirus 2014–2019" (PDF). Archived (PDF) from the original on 3 February 2019. Retrieved 3 February 2019.
- "Does polio still exist? Is it curable?". World Health Organization (WHO). Archived from the original on 29 May 2018. Retrieved 21 May 2018.
- "Poliomyelitis". World Health Organization (WHO). Archived from the original on 18 April 2017. Retrieved 25 April 2017.
- ^ "GPEI-nOPV2". Archived from the original on 27 July 2021. Retrieved 1 August 2021.
- ^ Fox M (20 April 2013). "Hilary Koprowski, Who Developed First Live-Virus Polio Vaccine, Dies at 96". The New York Times. Archived from the original on 25 August 2017. Retrieved 8 September 2017.
- Bazin H (2011). Vaccination: A History. John Libbey Eurotext. p. 395. ISBN 978-2742007752. Archived from the original on 8 September 2017.
- Smith DR, Leggat PA (2005). "Pioneering figures in medicine: Albert Bruce Sabin – inventor of the oral polio vaccine". The Kurume Medical Journal. 52 (3): 111–116. doi:10.2739/kurumemedj.52.111. PMID 16422178.
- World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Fine PE, Carneiro IA (November 1999). "Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative". American Journal of Epidemiology. 150 (10): 1001–1021. doi:10.1093/oxfordjournals.aje.a009924. PMID 10568615.
- Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, et al. (February 1991). "Transgenic mice susceptible to poliovirus". Proc. Natl. Acad. Sci. U.S.A. 88 (3): 951–955. Bibcode:1991PNAS...88..951K. doi:10.1073/pnas.88.3.951. PMC 50932. PMID 1846972.
- ^ Robertson S. Module 6: Poliomyelitis (PDF). The Immunological Basis for Immunization Series. World Health Organization (WHO). WHO/EPI/GEN/93.16. Archived from the original (PDF) on 19 October 2019. Retrieved 18 October 2019.
- Public Health Agency of Canada (18 July 2007). "Poliomyelitis vaccine: Canadian Immunization Guide". www.canada.ca. Archived from the original on 27 July 2021. Retrieved 1 August 2021.
- "Polio Vaccination: What Everyone Should Know | CDC". www.cdc.gov. 25 March 2021. Archived from the original on 16 January 2021. Retrieved 1 August 2021.
- Wahid R, Cannon MJ, Chow M (May 2005). "Virus-Specific CD4+ and CD8+ Cytotoxic T-Cell Responses and Long-Term T-Cell Memory in Individuals Vaccinated against Polio". Journal of Virology. 79 (10): 5988–5995. doi:10.1128/JVI.79.10.5988-5995.2005. PMC 1091702. PMID 15857985.
- ^ Atkinson W, Hamborsky J, McIntyre L, Wolfe S, eds. (2008). Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (PDF) (10th ed. (2nd printing) ed.). Washington, D.C.: Public Health Foundation. Archived from the original (PDF) on 24 September 2008. Retrieved 29 November 2008.
- ^ "Poliomyelitis prevention: recommendations for use of inactivated poliovirus vaccine and live oral poliovirus vaccine. American Academy of Pediatrics Committee on Infectious Diseases". Pediatrics. 99 (2): 300–305. February 1997. doi:10.1542/peds.99.2.300. PMID 9024465.
- Nathanson N, Martin JR (December 1979). "The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance". American Journal of Epidemiology. 110 (6): 672–692. doi:10.1093/oxfordjournals.aje.a112848. PMID 400274.
- ^ "OPV - Oral Polio Vaccine". Global Polio Eradication Initiative (GPEI). Retrieved 9 August 2024.
- Kunasekaran M. "Eradication of polio – Is Syria being left behind?". UNSW School of Public Health and Community Medicine. Archived from the original on 6 October 2018. Retrieved 6 October 2018.
- Marin M, Patel M, Oberste S, Pallansch MA (January 2017). "Guidance for Assessment of Poliovirus Vaccination Status and Vaccination of Children Who Have Received Poliovirus Vaccine Outside the United States". MMWR. Morbidity and Mortality Weekly Report. 66 (1): 23–25. doi:10.15585/mmwr.mm6601a6. PMC 5687270. PMID 28081056.
- "Poliomyelitis". World Health Organization (WHO). 22 July 2019. Archived from the original on 17 October 2019. Retrieved 18 October 2019.
- "GPEI Strategy for the Response to cVDPV2 2020–2021" (PDF). Polio Global Eradication Initiative. Archived (PDF) from the original on 1 August 2021. Retrieved 1 August 2021.
- "GPEI-OPV Oral polio vaccine". Global Polio Eradication Initiative - World Health Organization. 12 April 2024. Retrieved 12 April 2024.
- "GPEI-OPV Oral polio vaccine". Global Polio Eradication Initiative - World Health Organization. 12 April 2024. Retrieved 12 April 2024.
- Gadye L (14 June 2023). "Two New Vaccines Join the Fight to Eradicate Polio | UC San Francisco". The University of California San Francisco. Retrieved 10 August 2024.
- "Share of one-year-olds vaccinated against polio (Pol3)". Our World in Data. Archived from the original on 22 February 2020. Retrieved 4 March 2020.
- "Common and Rare Side Effects for poliovirus vaccine injection". www.webmd.com. Archived from the original on 24 August 2021. Retrieved 24 August 2021.
- Shimizu H, Thorley B, Paladin FJ, Brussen KA, Stambos V, Yuen L, et al. (December 2004). "Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001". Journal of Virology. 78 (24): 13512–13521. doi:10.1128/JVI.78.24.13512-13521.2004. PMC 533948. PMID 15564462.
- "nOPV2: Clinical Development and Evidence Summary" (PDF). Global Polio Eradication Initiative. April 2023. Retrieved 10 August 2024.
- ^ "Simian Virus 40 (SV40), Polio Vaccine, and Cancer". Vaccine Safety. Centers for Disease Control. 22 April 2004. Archived from the original on 22 May 2013. Retrieved 22 May 2013.
- Eddy BE, Borman GS, Berkeley WH, Young RD (May 1961). "Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts". Proceedings of the Society for Experimental Biology and Medicine. 107: 191–197. doi:10.3181/00379727-107-26576. PMID 13725644. S2CID 31275908.
- Carbone M (December 1999). "Simian virus 40 and human tumors: It is time to study mechanisms". Journal of Cellular Biochemistry. 76 (2): 189–193. doi:10.1002/(SICI)1097-4644(20000201)76:2<189::AID-JCB3>3.0.CO;2-J. PMID 10618636. S2CID 795975.
- Vilchez RA, Kozinetz CA, Arrington AS, Madden CR, Butel JS (June 2003). "Simian virus 40 in human cancers". The American Journal of Medicine. 114 (8): 675–684. doi:10.1016/S0002-9343(03)00087-1. PMID 12798456.
- ^ Engels EA (April 2005). "Cancer risk associated with receipt of vaccines contaminated with simian virus 40: epidemiologic research". Expert Review of Vaccines. 4 (2): 197–206. doi:10.1586/14760584.4.2.197. PMID 15889993. S2CID 5861910. Archived from the original on 20 April 2020. Retrieved 30 June 2019.
- Bookchin D (7 July 2004). "Vaccine scandal revives cancer fear". New Scientist. Archived from the original on 20 July 2004. Retrieved 29 November 2008.
- Strickler HD, Rosenberg PS, Devesa SS, Hertel J, Fraumeni JF, Goedert JJ (January 1998). "Contamination of poliovirus vaccines with simian virus 40 (1955–1963) and subsequent cancer rates". JAMA. 279 (4): 292–295. doi:10.1001/jama.279.4.292. PMID 9450713.
- Olin P, Giesecke J (1998). "Potential exposure to SV40 in polio vaccines used in Sweden during 1957: no impact on cancer incidence rates 1960 to 1993". Developments in Biological Standardization. 94: 227–233. PMID 9776244.
- ^ "Competition to develop an oral vaccine". Conquering Polio. Sanofi Pasteur SA. 2 February 2007. Archived from the original on 7 October 2007.
- ^ Plotkin SA (April 2001). "CHAT oral polio vaccine was not the source of human immunodeficiency virus type 1 group M for humans". Clinical Infectious Diseases. 32 (7): 1068–1084. doi:10.1086/319612. PMID 11264036.
- ^ Koprowski H (July 1960). "Historical aspects of the development of live virus vaccine in poliomyelitis". British Medical Journal. 2 (5192): 85–91. doi:10.1136/bmj.2.5192.85. PMC 2096806. PMID 14410975.
- Collins H (6 November 2000). "The Gulp Heard Round the World". The Philadelphia Inquirer. p. D-1. Archived from the original on 5 April 2004. Retrieved 29 November 2008.
- "Nigeria Muslims oppose polio vaccination". BBC News Online. 27 June 2002. Archived from the original on 29 November 2008. Retrieved 29 November 2008.
- Dugger CW, McNeil DG (20 March 2006). "Rumor, Fear and Fatigue Hinder Final Push to End Polio". The New York Times. Archived from the original on 10 December 2008. Retrieved 29 November 2008.
- "Anti-polio vaccine Malians jailed". BBC News Online. 12 May 2005. Archived from the original on 10 January 2006. Retrieved 29 November 2008.
- ^ Jegede AS (March 2007). "What led to the Nigerian boycott of the polio vaccination campaign?". PLOS Medicine. 4 (3): e73. doi:10.1371/journal.pmed.0040073. PMC 1831725. PMID 17388657.
- ^ Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA (2005). "Vaccine-derived polioviruses and the endgame strategy for global polio eradication". Annual Review of Microbiology. 59: 587–635. doi:10.1146/annurev.micro.58.030603.123625. PMID 16153180. Archived from the original on 9 July 2020. Retrieved 30 June 2019.
- ^ Joint Committee on Vaccination and Immunisation (2006). "26: Poliomyelitis" (PDF). In Salisbury D, Ramsay M, Noakes K (eds.). Immunisation Against Infectious Disease. Edinburgh: Stationery Office. pp. 313–329. ISBN 978-0-11-322528-6. Archived from the original (PDF) on 15 June 2007.
- ^ Sabin AB, Ramos-Alvarez M, Alvarez-Amezquita J, Pelon W, Michaels RH, Spigland I, et al. (August 1960). "Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses". JAMA. 173 (14): 1521–1526. doi:10.1001/jama.1960.03020320001001. PMID 14440553.
- Sabin AB (1987). "Role of my cooperation with Soviet scientists in the elimination of polio: possible lessons for relations between the U.S.A. and the USSR". Perspectives in Biology and Medicine. 31 (1): 57–64. doi:10.1353/pbm.1987.0023. PMID 3696960. S2CID 45655185.
- Benison S (1982). "International medical cooperation: Dr. Albert Sabin, live poliovirus vaccine and the Soviets". Bulletin of the History of Medicine. 56 (4): 460–483. PMID 6760938.
- Ochs K, Zeller A, Saleh L, Bassili G, Song Y, Sonntag A, et al. (January 2003). "Impaired binding of standard initiation factors mediates poliovirus translation attenuation". Journal of Virology. 77 (1): 115–122. doi:10.1128/JVI.77.1.115-122.2003. PMC 140626. PMID 12477816.
- Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E (February 1999). "Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence". Journal of Virology. 73 (2): 958–964. doi:10.1128/JVI.73.2.958-964.1999. PMC 103915. PMID 9882296.
- Pearce JM (November 2004). "Salk and Sabin: poliomyelitis immunisation". Journal of Neurology, Neurosurgery, and Psychiatry. 75 (11): 1552. doi:10.1136/jnnp.2003.028530. PMC 1738787. PMID 15489385.
- Smallman-Raynor M (2006). Poliomyelitis: A World Geography: Emergence to Eradication. Oxford University Press (US). ISBN 978-0-19-924474-4.
- Poliomyelitis Eradication: Field Guide. Washington: Pan American Health Organization. 2006. ISBN 978-92-75-11607-4.
- ^ Löwy I (1 April 2006). "Book Review". Medical History. 50 (2): 253–254. doi:10.1017/S0025727300009790 (inactive 1 November 2024). ISSN 0025-7273. PMC 1472109.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Rivers T (1967). Tom Rivers: reflections on a life in medicine and science: an oral history memoir. MIT Press. hdl:2027/heb.05734. ISBN 978-0262020268.
- ^ Halpern SA (2006). Lesser harms: The morality of risk in medical research. Chicago, Ill: University of Chicago Press. OCLC 877210630.
- ^ Hovern D (2018). The Trials and Triumphs of the American Polio Vaccine (Thesis). Cooper Medical School of Rowan University. Archived from the original on 21 July 2021. Retrieved 19 July 2021.
- "Vaccine Testing and Vulnerable Human Subjects | History of Vaccines". www.historyofvaccines.org. Archived from the original on 2 August 2021. Retrieved 18 July 2021.
- ^ Gould T (1997). A Summer Plague: Polio and Its Survivors. New Haven: Yale University Press. ISBN 978-0300072761. OCLC 38243151.
- Wilson JR (1963). Margin of Safety: The Story of Poliomyelitis Vaccine. Garden City, NY: Collins. OCLC 630735949.
- Paul, J.R. (1971). A History of Poliomyelitis. New Haven: Yale University Press.
- ^ Berk LB (1989). "Polio Vaccine Trials of 1935". Transactions & Studies of the College of Physicians of Philadelphia. 11 (4): 321–336. PMID 2692236.
- ^ Offit PA (2007). The Cutter Incident: How America's First Polio Vaccine Led to the Growing Vaccine Crisis. Yale University Press. p. 38. ISBN 978-0-300-12605-1.
- ^ Broadie M, Park W (5 October 1935). "Active immunization against poliomyelitis". Journal of the American Medical Association. 105 (14): 1089–1093. doi:10.1001/jama.1935.02760400005002. S2CID 1640997. Archived from the original on 19 July 2021. Retrieved 19 July 2021.
- Brodie M (1935). "Active immunization in monkeys against poliomyelitis with germicidally inactivated virus". The Journal of Immunology. 28 (1): 1–18. doi:10.4049/jimmunol.28.1.1.
- ^ Johnston K (22 February 2021). "The tragic story of a Canadian vaccine trailblazer". Macleans.ca. Archived from the original on 19 July 2021. Retrieved 18 July 2021.
- Oshinsky DM (2005). Polio: An American Story. Oxford University Press. p. 57. ISBN 978-0-19-515294-4. Archived from the original on 25 November 2023. Retrieved 23 March 2022.
- ^ "Poliomyelitis". American Journal of Public Health and the Nation's Health. 26 (2): 181–183. February 1936. doi:10.2105/AJPH.26.2.181. PMC 1562619. PMID 18014373.
- "John A. Kolmer, M.D." Archived from the original on 1 December 2017. Retrieved 28 November 2017.
- Enders JF, Weller TH, Robbins FC (January 1949). "Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues". Science. 109 (2822): 85–87. Bibcode:1949Sci...109...85E. doi:10.1126/science.109.2822.85. PMID 17794160.
- "The Nobel Prize in Physiology or Medicine 1954". The Nobel Foundation. Archived from the original on 19 December 2008. Retrieved 29 November 2008.
- Hammon WM, Coriell LL, Wehrle PF, Stokes J (April 1953). "Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. IV. Final report of results based on clinical diagnoses". Journal of the American Medical Association. 151 (15): 1272–1285. PMID 13034471.
- Ochmann S, Roser M (9 November 2017). "Polio – Polio Cases (OWID based on US Public Health Service (1910–1951) and US Center for Disease Control (1960–2010))". Our World in Data. Archived from the original on 28 March 2018. Retrieved 26 March 2018.
- "Public Health Weekly Reports for October 10, 1947". Public Health Reports. 62 (41): 1467–1498. October 1947. PMC 1995293. PMID 19316151.
- Koprowski H (15 October 2010). "Interview with Hilary Koprowski, sourced at History of Vaccines website". College of Physicians of Philadelphia. Archived from the original on 15 May 2016. Retrieved 15 October 2010.
- "science.ca : Leone N. Farrell". www.science.ca. Archived from the original on 28 June 2020. Retrieved 1 August 2021.
- Elliott CK (March 2011). "Leone Norwood Farrell, PhD". polioplace. Post-polio Health International. Archived from the original on 9 August 2019. Retrieved 10 August 2019.
- Shors T (2008). Understanding viruses. Jones & Bartlett Learning. pp. 294–. ISBN 978-0-7637-2932-5. Archived from the original on 21 July 2014. Retrieved 22 February 2011.
- Conis E (2016). "Political Ills". Distillations. 2 (2): 34–37. Archived from the original on 20 March 2018. Retrieved 27 March 2018.
- Oshinsky D (Winter 2010). "Miracle Workers". American Heritage. Archived from the original on 3 September 2014. Retrieved 1 September 2014.
- "Polio Victory Remembered as March of Dimes Marks 50th Anniversary of Salk Vaccine Field Trials". News Desk. 26 April 2004. Archived from the original on 27 September 2015. Retrieved 14 November 2014.
- Smith JS (1990). Patenting the Sun: Polio and the Salk Vaccine. William Morrow & Co. ISBN 978-0-688-09494-2.
- Sorem A, Sass EJ, Gottfried G (1996). Polio's legacy: an oral history. Washington, D.C.: University Press of America. ISBN 978-0-7618-0144-3.
- Hinman AR (June 1984). "Landmark perspective: Mass vaccination against polio". JAMA. 251 (22): 2994–2996. doi:10.1001/jama.1984.03340460072029. PMID 6371280.
- The Pasteur Institute stated that an anti poliomyelitis vaccine, developed by Professor Pierre Lepine would soon be produced in large quantities. (Times, London, 4 April 1955).
- "Pierre Lépine (1901–1989) – Notice biographique". Archives de l'Institut Pasteur. Archived from the original on 28 November 2016. Retrieved 28 November 2016.
- ^ Jacobs CD (2015). Jonas Salk: A Life. New York, NY: Oxford University Press. OCLC 919967059.
- "Technical Report on Poliomyelitis Vaccine". Public Health Reports. 70 (8): 742. August 1955.
- Juskewitch JE, Tapia CJ, Windebank AJ (August 2010). "Lessons from the Salk polio vaccine: methods for and risks of rapid translation". Clinical and Translational Science (Review). 3 (4): 182–185. doi:10.1111/j.1752-8062.2010.00205.x. PMC 2928990. PMID 20718820.
- ^ "Two Vaccines: Sabin and Salk". Smithsonian National Museum of American History. 27 September 2021. Archived from the original on 20 January 2017. Retrieved 24 April 2017.
- "Sabin receives highest Soviet civilian honor". U.P.I. archives. 20 November 1986. Archived from the original on 25 April 2017. Retrieved 25 April 2017.
- Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus Vaccine Combined
- ^ "Pediarix". U.S. Food and Drug Administration (FDA). 6 November 2019. Archived from the original on 22 September 2019. Retrieved 8 July 2020.
- "FDA Statistical Review and Evaluation" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original on 6 August 2020. Retrieved 8 July 2020.
- Mansoor H (23 October 2015). "Can India's social mobilisation strategy work in Pakistan?". The Express Tribune. Archived from the original on 24 October 2015. Retrieved 24 October 2015.
- Mastny L (25 January 1999). "Eradicating Polio: A Model for International Cooperation". Worldwatch Institute. Archived from the original on 3 December 2006. Retrieved 29 November 2008.
- ^ Centers for Disease Control Prevention (CDC) (October 1994). "Certification of poliomyelitis eradication--the Americas, 1994". MMWR. Morbidity and Mortality Weekly Report. 43 (39). Centers for Disease Control and Prevention: 720–722. PMID 7522302. Archived from the original on 21 May 2017.
- Yamazaki S, Toraya H (2001). "General News. Major Milestone reached in Global Polio Eradication: Western Pacific Region is certified Polio-Free". Health Educ Res. 16 (1): 109–114. Bibcode:2001PDiff..16..110Y. doi:10.1093/her/16.1.109.
- D' Souza RM, Kennett M, Watson C (2002). "Australia declared polio-free". Communicable Diseases Intelligence Quarterly Report. 26 (2): 253–260. PMID 12206379.
- "Europe achieves historic milestone as Region is declared polio-free" (Press release). World Health Organization (WHO). 21 June 2002. Archived from the original on 16 September 2008. Retrieved 23 August 2008.
- Ray K (26 February 2012). "India wins battle against dreaded polio". Deccan Herald.
- "India polio-free for a year: 'First time in history we're able to put up such a map'". The Telegraph. 26 February 2012. Archived from the original on 27 February 2012. Retrieved 26 February 2012.
- "India three years polio-free". World Health Organization (WHO). Archived from the original on 1 March 2017. Retrieved 19 February 2017.
- Barron L (4 November 2013). "Armenian Health Ministry: Syrian Armenian children need polio vaccine". CISTran Finance. Archived from the original on 19 December 2013. Retrieved 18 December 2013.
- De Vivo DC, Ryan MM, Jones HR, Darras BT (2014). Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician's Approach. Elsevier Science. p. 161. ISBN 978-0-12-417127-5. Archived from the original on 23 April 2017.
- Charles Kurzman. The Missing Martyrs. Oxford University Press. p. 130.
Resistance to polio vaccination is a fringe position. Almost every Muslim scholar and political leader has endorsed the vaccine, and all but a few Muslim-majority countries have wiped out the disease entirely.
- ^ "Pakistan polio official killed in Peshawar: police". The Daily Star: Lebanon. 11 September 2016. Archived from the original on 11 September 2016. Retrieved 11 September 2016.
- Najafizada E (15 December 2015). "Taliban Join Global Effort to Kill Off Polio in 2016". Bloomberg.com. Archived from the original on 28 February 2017.
- "International Travel and Health. Chapter 6 – Vaccine-preventable diseases and vaccines (2019 update)" (PDF). World Health Organization. United Nations. 2020. Archived (PDF) from the original on 11 April 2020. Retrieved 2 December 2020.
- ^ "Countries with risk of yellow fever transmission and countries requiring yellow fever vaccination (July 2019)". World Health Organization. United Nations. 4 July 2019. Archived from the original on 27 January 2021. Retrieved 2 December 2020.
- Scherbel-Ball N (25 August 2020). "Africa declared free of polio". BBC News. Archived from the original on 26 August 2020. Retrieved 25 August 2020.
- "Key At-Risk Countries". Global Polio Eradication Initiative. World Health Organization. Archived from the original on 29 April 2021. Retrieved 2 December 2020.
- "Afghanistan Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 1 October 2020. Archived from the original on 21 October 2020. Retrieved 2 December 2020.
- "Belize Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Brunei Darussalam Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 1 December 2020. Retrieved 2 December 2020.
- "Egypt Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 8 March 2021. Retrieved 2 December 2020.
- "Georgia Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 17 January 2021. Retrieved 2 December 2020.
- "India Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Iran Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Iraq Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 20 January 2021. Retrieved 2 December 2020.
- "Jordan Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Lebanon Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Libya Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Maldives Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Morocco Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 21 October 2020. Retrieved 2 December 2020.
- "Nepal Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 21 September 2020. Retrieved 2 December 2020.
- "Oman Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 15 August 2020. Retrieved 2 December 2020.
- "Pakistan Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 21 October 2020. Retrieved 2 December 2020.
- "Philippines Recommended Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 25 October 2020. Retrieved 2 December 2020.
- "Qatar Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 2 December 2020. Retrieved 2 December 2020.
- "Saint Kitts & Nevis Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 4 December 2020. Retrieved 2 December 2020.
- "Saudi Arabia Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 27 February 2021. Retrieved 2 December 2020.
- "Seychelles Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 4 August 2020. Retrieved 2 December 2020.
- "Syria Required Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 30 October 2020. Retrieved 2 December 2020.
- "Ukraine Recommended Vaccinations: Polio". iamat.org. International Association for Medical Assistance to Travellers (IAMAT). 23 October 2020. Archived from the original on 24 January 2021. Retrieved 2 December 2020.
- "Availability and price of inactivated polio vaccine". The Global Polio Eradication Initiative. Archived from the original on 11 April 2015.
- "Impotence fears hit polio drive". BBC News Online. 25 January 2007. Archived from the original on 9 October 2015. Retrieved 15 December 2015.
- Junaidi I (14 January 2015). "Lab tests show polio vaccine is not 'Haram'". dawn.com. Archived from the original on 22 December 2015. Retrieved 15 December 2015.
Further reading
- Ramsay M, ed. (2013). "Polio: the green book, chapter 26". Immunisation against infectious disease. London: Public Health England. Archived from the original on 12 November 2019. Retrieved 23 December 2019.
- Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. (2021). "Chapter 18: Poliomyelitis". Epidemiology and Prevention of Vaccine-Preventable Diseases (14th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 30 December 2016. Retrieved 23 December 2019.
- Routh JA, Oberste MS, Patel M (2018). "Chapter 12: Poliomyelitis". In Roush SW, Baldy LM, Hall MH (eds.). Manual for the surveillance of vaccine-preventable diseases. Atlanta, Georgia: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 1 August 2020. Retrieved 23 December 2019.
External links
- "Polio Vaccine Information Statement". Centers for Disease Control and Prevention (CDC). August 2021.
- History of Vaccines Website – History of Polio History of Vaccines, a project of the College of Physicians of Philadelphia
- PBS.org – 'People and Discoveries: Salk Produces Polio Vaccine 1952', Public Broadcasting Service (PBS)
- "IPOL – Poliovirus Vaccine Inactivated (Monkey Kidney Cell)". U.S. Food and Drug Administration (FDA). 11 December 2019. STN: 103930.
- Poliovirus Vaccines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| Artificial induction of immunity / Immunization: Vaccines, Vaccination, Infection, Inoculation (J07) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Development | |||||||||||
| Classes | |||||||||||
| Administration | |||||||||||
| Vaccines |
| ||||||||||
| Inventors/ researchers | |||||||||||
| Controversy | |||||||||||
| Related | |||||||||||
| |||||||||||