| Revision as of 01:47, 4 May 2017 editInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,387,723 edits Rescuing 1 sources and tagging 0 as dead. #IABot (v1.3beta8)← Previous edit | Latest revision as of 07:57, 25 December 2024 edit undoMeters (talk | contribs)Extended confirmed users, New page reviewers, Pending changes reviewers, Rollbackers172,687 editsm Reverted edit by 202.126.91.227 (talk) to last version by QuondumTag: Rollback | ||
| Line 1: | Line 1: | ||
| <!-- Do not use: {{lowercase}} - kelvin (temperature) is treated as a common noun, capitalized in TITLES and start of a sentence --> | |||
| {{about|the unit of temperature}} | |||
| {{Short description|SI unit of temperature}} | |||
| {{pp-move-indef}} | |||
| {{temperature}} | {{About|the unit of temperature|the person|Lord Kelvin|other uses}} | ||
| {{Use dmy dates|date=December 2020|cs1-dates=y}} | |||
| The '''kelvin''' is a ] for ] based upon an ]. It is one of the seven base units in the ] (SI) and is assigned the unit symbol '''K'''. The '''Kelvin scale''' is an absolute, ] ] using as its null point ], the temperature at which all ] ceases in the classical description of ]. The kelvin is defined as the fraction {{frac|273.16}} of the thermodynamic temperature of the ] (exactly 0.01 °C or 32.018 °F).<ref name=res413>{{cite web |title=Resolution 4: Definition of the SI unit of thermodynamic temperature (kelvin) |work=Resolutions of the 13th CGPM |url=http://www.bipm.fr/en/CGPM/db/13/4/ |publisher=Bureau International des Poids et Mesures |year=1967 |accessdate=2008-02-06}}</ref> In other words, it is defined such that the ] of water is exactly 273.16 K. | |||
| {{Use British English|date=November 2018}} | |||
| {{Infobox Unit | |||
| | name = kelvin <!--not capitalized--> | |||
| | image = Temperature-scales-comparison.svg | |||
| | caption = Equivalent temperatures in kelvin (K), Celsius (°C), and ] (°F) | |||
| | standard = ] | |||
| | quantity = temperature | |||
| | symbol = K | |||
| | namedafter = ] | |||
| | convertfromx = yes | |||
| | units1 = ] | |||
| | inunits1 = ({{Math|''x''}} − 273.15) °C | |||
| | units2 = ]/] units | |||
| | inunits2 = (1.8{{Math|''x''}} − 459.67) ] | |||
| | units3 = Imperial/US absolute scale | |||
| | inunits3 = 1.8{{Math|''x''}} ] | |||
| | extraheader = | |||
| | extralabel = 2019 definition | |||
| | extradata = ] ≝ {{val|1.380649|e=-23}} {{sfrac|]|K}} | |||
| }} | |||
| The '''kelvin''' (symbol: '''K''') is the ] for ] in the ] (SI). The '''Kelvin scale''' is an ] ] that starts at the lowest possible temperature (]), taken to be 0 K.<ref name="MEP for kelvin 2019"/><ref name="SI Brochure 9"/><ref name="BIPM web page for kelvin"/><ref name="NIST SI redefinition"/> By definition, the ] scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15.<ref name="MEP for kelvin 2019" /><ref name="nist intro" /> | |||
| The Kelvin scale is named after the Belfast-born, ] engineer and physicist ] (1824–1907), who wrote of the need for an "absolute thermometric scale". Unlike the degree ] and degree ], the kelvin is not referred to or typeset as a ]. The kelvin is the primary unit of temperature measurement in the physical sciences, but is often used in conjunction with the Celsius degree, which has the same magnitude. The definition implies that absolute zero (0 K) is equivalent to {{convert|-273.15|C|F}}. | |||
| The 19th century British scientist ] first developed and proposed the scale.<ref name="nist intro"/> It was often called the "absolute Celsius" scale in the early 20th century.<ref name="absoluteC"/> The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the ]. The Celsius, ], and ] scales were redefined in terms of the Kelvin scale using this definition.<ref name="SI Brochure 9" /><ref name="Busting Myths about the Metric System" /><ref name="NIST HB44 Appendix C" /> The ] now defines the kelvin in terms of energy by setting the ] to ''exactly'' {{physconst|k|ref=no|symbol=no|unit=] per kelvin}};<ref name="SI Brochure 9" /> every 1 K change of ] corresponds to a ] change of exactly {{val|1.380649|e=−23|u=J}}. | |||
| ==History== | |||
| == History == | |||
| {{see also|Thermodynamic temperature#History}} | {{see also|Thermodynamic temperature#History}} | ||
| ], the namesake of the unit]] | |||
| === Precursors === | |||
| In 1848, Lord Kelvin wrote in his paper, ''On an Absolute Thermometric Scale'', of the need for a scale whereby "infinite cold" (absolute zero) was the scale's null point, and which used the degree Celsius for its unit increment. Kelvin calculated that absolute zero was equivalent to −273 °C on the air thermometers of the time.<ref name=lordkelvin>{{cite journal |last=Lord Kelvin|first=William |title=On an Absolute Thermometric Scale |journal=Philosophical Magazine |date=October 1848 |url=http://zapatopi.net/kelvin/papers/on_an_absolute_thermometric_scale.html |accessdate=2008-02-06}}</ref> This absolute scale is known today as the Kelvin thermodynamic temperature scale. Kelvin's value of "−273" was the negative reciprocal of 0.00366—the accepted expansion coefficient of gas per degree Celsius relative to the ice point, giving a remarkable consistency to the currently accepted value. | |||
| ] point for ] (shown here in Celsius) before the physical nature of heat was well understood.]] | |||
| During the 18th century, ] were developed,<ref name="NIST kelvin history">{{cite journal |title=Kelvin: History |url=https://www.nist.gov/si-redefinition/kelvin-history |journal=NIST |date=14 May 2018 |access-date=21 February 2022}}</ref> notably ] and centigrade (later Celsius). These scales predated much of the modern science of ], including ] and the ] which underpin the concept of absolute zero. Instead, they chose defining points within the range of human experience that could be reproduced easily and with reasonable accuracy, but lacked any deep significance in thermal physics. In the case of the Celsius scale (and the long since defunct ] and ]) the melting point of ice served as such a starting point, with Celsius being defined (from the ] to the ]) by calibrating a thermometer such that: | |||
| In 1954, Resolution 3 of the 10th ] (CGPM) gave the Kelvin scale its modern definition by designating the ] as its second defining point and assigned its temperature to exactly 273.16 kelvins.<ref name=res310>{{cite web |title=Resolution 3: Definition of the thermodynamic temperature scale |work=Resolutions of the 10th CGPM |publisher=Bureau International des Poids et Mesures |url=http://www.bipm.fr/en/CGPM/db/10/3/ |year=1954 |accessdate=2008-02-06}}</ref> | |||
| * Water's ] is 0 °C. | |||
| * Water's ] is 100 °C. | |||
| This definition assumes pure water at a specific ] chosen to approximate the natural air pressure at sea level. Thus, an increment of 1 °C equals {{sfrac|1|100}} of the temperature difference between the melting and boiling points. The same temperature interval was later used for the Kelvin scale. | |||
| In 1967/1968 Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature "kelvin", symbol K, replacing "degree Kelvin", symbol °K.<ref name=res313/> Furthermore, feeling it useful to more explicitly define the magnitude of the unit increment, the 13th CGPM also held in Resolution 4 that "The kelvin, unit of thermodynamic temperature, is equal to the fraction {{frac|273.16}} of the thermodynamic temperature of the ]."<ref name="res413" /> | |||
| === Charles's law === | |||
| In 2005 ] (CIPM), a committee of the CGPM, affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the Kelvin thermodynamic temperature scale would refer to water having an isotopic composition specified as ].<ref name=sib2115>{{cite web|title=Unit of thermodynamic temperature (kelvin) |work=SI Brochure, 8th edition |pages=Section 2.1.1.5 |url=http://www1.bipm.org/en/si/si_brochure/chapter2/2-1/2-1-1/kelvin.html |publisher=Bureau International des Poids et Mesures |year=1967 |accessdate=2008-02-06 |deadurl=yes |archiveurl=https://web.archive.org/web/20070926215600/http://www1.bipm.org/en/si/si_brochure/chapter2/2-1/2-1-1/kelvin.html |archivedate=26 September 2007 |df= }}</ref> | |||
| From 1787 to 1802, it was determined by ] (unpublished), ],<ref name="Dalton_1801_1"/><ref name="Dalton_1801_2"/> and ]<ref name="Gay-Lussac_1802"/> that, at constant pressure, ideal gases expanded or contracted their volume linearly (]) by about 1/273 parts per degree Celsius of temperature's change up or down, between 0 °C and 100 °C. Extrapolation of this law suggested that a gas cooled to about −273 °C would occupy zero volume. | |||
| == |
=== Lord Kelvin === | ||
| ], the namesake of the unit of measure.]] | |||
| When spelled out or spoken, the unit is pluralised using the same grammatical rules as for other ] such as the ] or ] (e.g. "the triple point of water is exactly 273.16 kelvins"<ref name=sib211>{{cite web |title=Rules and style conventions for expressing values of quantities |work=SI Brochure, 8th edition |pages=Section 2.1.1.5 |url=http://www.bipm.org/en/si/si_brochure/chapter2/2-1/kelvin.html |publisher=Bureau International des Poids et Mesures |year=1967 |accessdate=2012-08-27}}</ref>). When reference is made to the "Kelvin ''scale''", the word "kelvin"—which is normally a noun—functions ] to modify the noun "scale" and is capitalized. As with most other SI unit symbols (angle symbols, e.g. 45°3′4″, are the exception) there is a space between the numeric value and the kelvin symbol (e.g. "99.987 K").<ref name=nist>{{cite web |title=SI Unit rules and style conventions |url=http://physics.nist.gov/cuu/Units/checklist.html |publisher=National Institute of Standards and Technology |date=September 2004 |accessdate=2008-02-06}}</ref><ref name=sib533>{{cite web |title=Rules and style conventions for expressing values of quantities |work=SI Brochure, 8th edition |pages=Section 5.3.3 |url=http://www.bipm.org/en/publications/si-brochure/section5-3.html |publisher=Bureau International des Poids et Mesures |year=1967 |accessdate=2015-12-13}}</ref> | |||
| ==== First absolute scale ==== | |||
| Before the 13th CGPM in 1967–1968, the unit kelvin was called a "degree", the same as with the other temperature scales at the time. It was distinguished from the other scales with either the adjective suffix "Kelvin" ("degree Kelvin") or with "absolute" ("degree absolute") and its symbol was °K. The latter term (degree absolute), which was the unit's official name from 1948 until 1954, was ambiguous since it could also be interpreted as referring to the ]. Before the 13th CGPM, the plural form was "degrees absolute". The 13th CGPM changed the unit name to simply "kelvin" (symbol K).<ref>{{cite journal |author=Barry N. Taylor |title=Guide for the Use of the International System of Units (SI) |version=Special Publication 811 |format=.PDF |publisher=National Institute of Standards and Technology |url=http://physics.nist.gov/cuu/pdf/sp811.pdf |year=2008 |accessdate=2011-03-05}}</ref> The omission of "degree" indicates that it is not relative to an arbitrary reference point like the Celsius and Fahrenheit scales (although the Rankine scale continued to use "degree Rankine"), but rather an absolute unit of measure which can be manipulated algebraically (e.g. multiplied by two to indicate twice the amount of "mean energy" available among elementary degrees of freedom of the system). | |||
| In 1848, William Thomson, who was later ] as ], published a paper ''On an Absolute Thermometric Scale''.{{sfn|Thomson|1882|pp=100–106}} The scale proposed in the paper turned out to be unsatisfactory, but the principles and formulas upon which the scale was based were correct.<ref name="Magie">{{cite book |last1=Magie |first1=William Francis |title=A Source Book In Physics |date=1935 |page=237 |url=https://archive.org/details/in.ernet.dli.2015.449479/page/n251/mode/2up}}</ref> For example, in a footnote, Thomson derived the value of −273 °C for absolute zero by calculating the negative reciprocal of 0.00366—the ] of an ideal gas per degree Celsius relative to the ice point.<ref>{{harvnb|Thomson|1882|p=104}}: "If we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/·366, if ·366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low."</ref> This derived value agrees with the currently accepted value of −273.15 °C, allowing for the precision and uncertainty involved in the calculation. | |||
| <!--] provides a ] for the kelvin at U+212A (decimal 8490), for compatibility with CJK encodings that provide such a character (as such, in most fonts the width is the same as for ] characters).--> | |||
| The scale was designed on the principle that "a unit of heat descending from a body {{mvar|A}} at the temperature {{mvar|T}}° of this scale, to a body {{mvar|B}} at the temperature {{nowrap|({{mvar|T}} − 1)°}}, would give out the same mechanical effect, whatever be the number {{mvar|T}}."{{sfn|Thomson|1882|p=104}} Specifically, Thomson expressed the amount of work necessary to produce a unit of heat (the ]) as <math>\mu(t) (1 + E t)/E</math>, where <math>t</math> is the temperature in Celsius, <math>E</math> is the coefficient of thermal expansion, and <math>\mu(t)</math> was "Carnot's function", a substance-independent quantity depending on temperature,{{sfn|Thomson|1882|p=187}} motivated by an obsolete version of ].<ref name="Magie"/>{{sfn|Thomson|1882|p=106}} The scale is derived by finding a change of variables <math>T_{1848} = f(T)</math> of temperature <math>T</math> such that <math>dT_{1848} / dT</math> is proportional to <math>\mu</math>. | |||
| ===Use in conjunction with Celsius=== | |||
| ] | ] | ||
| When Thomson published his paper in 1848, he only considered Regnault's experimental measurements of <math>\mu(t)</math>.{{sfn|Thomson|1882|p=193}} That same year, ] suggested to Thomson that the true formula for Carnot's function was{{sfn|Thomson|1882|p=212}} | |||
| In science and engineering, degrees Celsius and kelvins are often used simultaneously in the same article, where absolute temperatures are given in degrees Celsius, but temperature intervals are given in kelvins. E.g. "its measured value was {{val|0.01028|u=°C}} with an uncertainty of 60 µK." | |||
| <math display="block">\mu(t) = J \frac{E}{1+E t},</math> | |||
| where <math>J</math> is "the mechanical equivalent of a unit of heat",{{sfn|Thomson|1882|p=186}} now referred to as the ] of water, approximately {{convert|771.8|ftlbf/F/lb|J/K/kg}}.{{sfn|Thomson|1882|p=192}} Thomson was initially skeptical of the deviations of Joule's formula from experiment, stating "I think it will be generally admitted that there can be no such inaccuracy in Regnault's part of the data, and there remains only the uncertainty regarding the density of saturated steam".{{sfn|Thomson|1882|pp=214–215}} Thomson referred to the correctness of Joule's formula as "]'s hypothesis", on account of it having been first assumed by Mayer.{{sfn|Thomson|1882|p=213}} Thomson arranged numerous experiments in coordination with Joule, eventually concluding by 1854 that Joule's formula was correct and the effect of temperature on the density of saturated steam accounted for all discrepancies with Regnault's data.{{sfn|Thomson|1882|p=388}} Therefore, in terms of the modern Kelvin scale <math>T</math>, the first scale could be expressed as follows:{{sfn|Thomson|1882|p=106}} | |||
| <math display="block">T_{1848} = 100 \times \frac{\log(T / \text{273 K})}{\log(\text{373 K} / \text{273 K})}</math> | |||
| The parameters of the scale were arbitrarily chosen to coincide with the Celsius scale at 0° and 100 °C or 273 and 373 K (the melting and boiling points of water).<ref>{{harvnb|Thomson|1882|p=105}}: "The arbitrary points which coincide on the two scales are 0° and 100°"</ref> On this scale, an increase of approximately 222 degrees corresponds to a doubling of Kelvin temperature, regardless of the starting temperature, and "infinite cold" (]) has a numerical value of negative ].<ref>{{cite journal |last1=Saslow |first1=WM |title=A History of Thermodynamics: The Missing Manual. |journal=Entropy |date=7 January 2020 |volume=22 |issue=1 |doi=10.3390/e22010077|doi-access=free |pmid=33285852|pmc=7516509 |bibcode=2020Entrp..22...77S |at=eqn. (36)}}</ref> | |||
| ==== Modern absolute scale ==== | |||
| This practice is permissible because the degree Celsius is a special name for the kelvin for use in expressing relative temperatures, and the magnitude of the degree Celsius is exactly equal to that of the kelvin.<ref name=sib222>{{cite web |title=Units with special names and symbols; units that incorporate special names and symbols |work=SI Brochure, 8th edition |pages=Section 2.2.2, Table 3 |url=http://www.bipm.org/en/si/si_brochure/chapter2/2-2/table3.html |publisher=Bureau International des Poids et Mesures |year=2006 |accessdate=2016-06-27}}</ref> Notwithstanding that the official endorsement provided by Resolution 3 of the 13th CGPM states "a temperature interval may also be expressed in degrees Celsius",<ref name=res313>{{cite web |title=Resolution 3: SI unit of thermodynamic temperature (kelvin) |work=Resolutions of the 13th CGPM |url=http://www.bipm.fr/en/CGPM/db/13/3/ |publisher=Bureau International des Poids et Mesures |year=1967 |accessdate=2008-02-06}}</ref> the practice of simultaneously using both "°C" and "K" is widespread throughout the scientific world. The use of ] forms of the degree Celsius (such as "µ°C" or "microdegrees Celsius") to express a temperature interval has not been widely adopted. | |||
| Thomson understood that with Joule's proposed formula for <math>\mu</math>, the relationship between work and heat for a perfect thermodynamic engine was simply the constant <math>J</math>.{{sfn|Thomson|1882|p=190|loc=formula (7)|}} In 1854, Thomson and Joule thus formulated a second absolute scale that was more practical and convenient, agreeing with air thermometers for most purposes.{{sfn|Thomson|1882|pp=106,232–236}} Specifically, "the numerical measure of temperature shall be simply the mechanical equivalent of the thermal unit divided by Carnot's function."{{sfn|Thomson|1882|p=234}} | |||
| ==Proposed redefinition== | |||
| {{main|Proposed redefinition of SI base units}} | |||
| To explain this definition, consider a reversible ] engine, where <math>Q_H</math> is the amount of heat energy transferred into the system, <math>Q_C</math> is the heat leaving the system, <math>W</math> is the work done by the system (<math>Q_H - Q_C</math>), <math>t_H</math> is the temperature of the hot reservoir in Celsius, and <math>t_C</math> is the temperature of the cold reservoir in Celsius. The Carnot function is defined as <math>\mu =W / Q_H / (t_H - t_C)</math>, and the absolute temperature as <math>T_H = J/\mu</math>. One finds the relationship <math>T_H= J \times Q_H \times (t_H-t_C)/W</math>. By supposing <math>T_H-T_C=J \times (t_H-t_c)</math>, one obtains the general principle of an absolute thermodynamic temperature scale for the Carnot engine, <math>Q_H / T_H = Q_C / T_C</math>. The definition can be shown to correspond to the thermometric temperature of the ]s.<ref>{{cite book |last1=Wang |first1=Lin-Shu |title=A treatise of heat and energy |date=2020 |publisher=Springer |location=Cham |isbn=9783030057466 |page=77}}</ref> | |||
| In 2005 the ] embarked on a program to redefine the kelvin (along with the other SI units) using a more experimentally rigorous methodology. The current definition {{asof|2016|lc=y}} is unsatisfactory for temperatures below 20 K and above {{val|1300|u=K}}.<ref name=nextdef>{{cite web |url=http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Report_to_CIPM_2.pdf |title=Report to the CIPM on the implications of changing the definition of the base unit kelvin |author=J. Fischer1|publisher=International Committee for Weights and Measures (CIPM) |date=2007-05-02 |accessdate=2010-02-23|display-authors=etal}}</ref> In particular, the committee proposed ] such that ] takes the exact value {{val|1.3806505|e=-23|u=J/K}}.<ref name="draft">{{cite web | |||
| This definition by itself is not sufficient. Thomson specified that the scale should have two properties:{{sfn|Thomson|1882|p=235}} | |||
| * The absolute values of two temperatures are to one another in the proportion of the heat taken in to the heat rejected in a perfect thermodynamic engine working with a source and refrigerator at the higher and lower of the temperatures respectively. | |||
| * The difference of temperatures between the freezing- and boiling-points of water under standard atmospheric pressure shall be called 100 degrees. (The same increment as the Celsius scale) Thomson's best estimates at the time were that the temperature of freezing water was 273.7 K and the temperature of boiling water was 373.7 K.{{sfn|Thomson|1882|p=236}} | |||
| These two properties would be featured in all future versions of the Kelvin scale, although it was not yet known by that name. In the early decades of the 20th century, the Kelvin scale was often called the "absolute ]" scale, indicating Celsius degrees counted from absolute zero rather than the freezing point of water, and using the same symbol for regular Celsius degrees, °C.<ref name="absoluteC">''Encyclopaedia Britannica'' editions from the 1920s and 1950s, the article "Planets".</ref> | |||
| === Triple point standard === | |||
| ]. The solid green line applies to most substances; the dashed green line gives the anomalous behavior of water. The boiling line (solid blue) runs from the triple point to the ], beyond which further increases in temperature and pressure produce a ].]] | |||
| In 1873, William Thomson's older brother ] coined the term '']''<ref>{{Cite journal|last=Thomson|first=James|date=1873|title=A quantitative investigation of certain relations between the gaseous, the liquid, and the solid states of water-substance|journal=Proceedings of the Royal Society of London|url=https://babel.hathitrust.org/cgi/pt?id=hvd.32044106377062&view=1up&seq=48|volume=22|page=28|bibcode=1873RSPS...22...27T |issn=0370-1662|quote=and consequently that the three curves would meet or cross each other in one point, which I have called the ''triple point''.}}</ref> to describe the combination of temperature and ] at which the solid, liquid, and gas ] of a substance were capable of coexisting in ]. While any two phases could coexist along a range of temperature-pressure combinations (e.g. the ] of water can be affected quite dramatically by raising or lowering the pressure), the triple point condition for a given substance can occur only at a single pressure and only at a single temperature. By the 1940s, the triple point of water had been experimentally measured to be about 0.6% of ] and very close to 0.01 °C per the historical definition of Celsius then in use. | |||
| In 1948, the Celsius scale was recalibrated by assigning the triple point temperature of water the value of 0.01 °C exactly<ref name=":0">{{Cite journal |last=Swinton |first=F. L. |date=September 1967 |title=The triplet point of water |url=https://pubs.acs.org/doi/abs/10.1021/ed044p541 |journal=Journal of Chemical Education |language=en |volume=44 |issue=9 |pages=541 |doi=10.1021/ed044p541 |bibcode=1967JChEd..44..541S |issn=0021-9584}}</ref> and allowing the ] at standard atmospheric pressure to have an empirically determined value (and the actual melting point at ambient pressure to have a ] value) close to 0 °C. This was justified on the grounds that the triple point was judged to give a more accurately reproducible reference temperature than the melting point.<ref name="CGPM 9 res 3 (1948)">{{cite web |title=Resolution 3 of the 9th CGPM (1948) |url=https://www.bipm.org/en/committees/cg/cgpm/9-1948/resolution-3 |publisher=BIPM |access-date=21 February 2022}}</ref> The triple point could be measured with ±0.0001 °C accuracy, while the melting point just to ±0.001 °C.<ref name=":0" /> | |||
| In 1954, with absolute zero having been experimentally determined to be about −273.15 °C per the definition of °C then in use, Resolution 3 of the 10th ] (CGPM) introduced a new internationally standardized Kelvin scale which defined the triple point as exactly 273.15 + 0.01 = 273.16 degrees Kelvin.<ref name="CGPM 10 res 3 (1954)">{{cite web |title=Resolution 3 of the 10th CGPM (1954) |url=https://www.bipm.org/en/committees/cg/cgpm/10-1954/resolution-3 |publisher=BIPM |access-date=21 February 2022}}</ref><ref name=res310>{{cite web |title=Resolution 3: Definition of the thermodynamic temperature scale |work=Resolutions of the 10th CGPM |publisher=Bureau International des Poids et Mesures |url=http://www.bipm.fr/en/CGPM/db/10/3/ |year=1954 |access-date=2008-02-06 |url-status=dead |archive-url=https://web.archive.org/web/20070623215318/http://www.bipm.fr/en/CGPM/db/10/3/ |archive-date=23 June 2007}}</ref> | |||
| In 1967/1968, Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature "kelvin", symbol K, replacing "degree Kelvin", symbol {{not a typo|°K}}.<ref name="CGPM 13 res 3 (1967)" /><ref name=res313>{{cite web |title=Resolution 3: SI unit of thermodynamic temperature (kelvin) |work=Resolutions of the 13th CGPM |url=http://www.bipm.fr/en/CGPM/db/13/3/ |publisher=Bureau International des Poids et Mesures |year=1967 |access-date=2008-02-06 |url-status=dead |archive-url=https://web.archive.org/web/20070421013852/http://www.bipm.fr/en/CGPM/db/13/3/ |archive-date=21 April 2007}}</ref><ref name="Westphal_1952"/> The 13th CGPM also held in Resolution 4 that "The kelvin, unit of thermodynamic temperature, is equal to the fraction {{sfrac|273.16}} of the thermodynamic temperature of the triple point of water."<ref name="NIST SI redefinition" /><ref name="CGPM 13 res 4 (1967)">{{cite web |title=Resolution 4 of the 13th CGPM (1967) |url=https://www.bipm.org/en/committees/cg/cgpm/13-1967/resolution-4 |publisher=BIPM |access-date=21 February 2022}}</ref><ref name="res413">{{cite web|url=https://www.bipm.org/en/CGPM/db/13/4/|title=Resolution 4: Definition of the SI unit of thermodynamic temperature (kelvin)|year=1967|work=Resolutions of the 13th CGPM|publisher=Bureau International des Poids et Mesures|archive-url=https://web.archive.org/web/20070615125646/http://www.bipm.fr/en/CGPM/db/13/4/|archive-date=15 June 2007|url-status=dead |access-date=2008-02-06}}</ref> | |||
| After ], this left the kelvin, the second, and the kilogram as the only SI units not defined with reference to any other unit. | |||
| In 2005, noting that the triple point could be influenced by the isotopic ratio of the hydrogen and oxygen making up a water sample and that this was "now one of the major sources of the observed variability between different realizations of the water triple point", the ] (CIPM), a committee of the CGPM, affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the kelvin would refer to water having the isotopic composition specified for ].<ref name="NIST SI redefinition" /><ref name="CGPM 23 res 10 (2007)">{{cite web |title=Resolution 10 of the 23rd CGPM (2007) |url=https://www.bipm.org/en/committees/cg/cgpm/23-2007/resolution-10 |publisher=BIPM |access-date=21 February 2022}}</ref><ref name=sib2115>{{cite web|title=Unit of thermodynamic temperature (kelvin) |work=SI Brochure, 8th edition |at=Section 2.1.1.5 |url=http://www1.bipm.org/en/si/si_brochure/chapter2/2-1/2-1-1/kelvin.html |publisher=Bureau International des Poids et Mesures |year=1967 |access-date=2008-02-06 |url-status=dead |archive-url=https://web.archive.org/web/20070926215600/http://www1.bipm.org/en/si/si_brochure/chapter2/2-1/2-1-1/kelvin.html |archive-date=26 September 2007 }}</ref> | |||
| === 2019 redefinition === | |||
| {{main|2019 revision of the SI}} | |||
| ]. The joule is not shown because it is a ] defined by the metre (m), second (s), and kilogram (kg). Those SI base units are themselves defined by the ] of the ] (''{{mvar|c}}''), the ] ({{math|Δ''ν''<sub>Cs</sub>}}) and the ] (''{{mvar|h}}''). Black arrows trace the dependencies from these constants to the kelvin.]] | |||
| In 2005, the ] began a programme to redefine the kelvin (along with other ]) using a more experimentally rigorous method. In particular, the committee proposed ] such that the ] ({{math|''k''<sub>B</sub>}}) would take the exact value {{val|1.3806505|e=-23|u=J|up=K}}.<ref name="draft"> | |||
| {{cite web | |||
| |url=http://www.bipm.org/utils/en/pdf/si_brochure_draft_ch2.pdf | |url=http://www.bipm.org/utils/en/pdf/si_brochure_draft_ch2.pdf | ||
| |title=Draft Chapter 2 for SI Brochure, following redefinitions of the base units | |title=Draft Chapter 2 for SI Brochure, following redefinitions of the base units | ||
| |author=Ian Mills | |author=Ian Mills | ||
| |publisher=CCU | |publisher=CCU | ||
| |website=BIPM | |||
| |date=29 September 2010 | |date=29 September 2010 | ||
| |access-date=2011-01-01 | |||
| |accessdate=2011-01-01}}</ref> The committee had hoped that the program would be completed in time for its adoption by the CGPM at its 2011 meeting, but at the 2011 meeting the decision was postponed to the 2014 meeting when it would be considered as part of a ].<ref>{{cite press release | |||
| |url-status=dead | |||
| |archive-url=https://web.archive.org/web/20110110104615/http://www.bipm.org/utils/en/pdf/si_brochure_draft_ch2.pdf | |||
| |archive-date=10 January 2011 | |||
| }}</ref> The committee hoped the program would be completed in time for its adoption by the CGPM at its 2011 meeting, but at the 2011 meeting the decision was postponed to the 2014 meeting when it would be considered part of a ].<ref> | |||
| {{cite press release | |||
| | url = http://www.bipm.org/utils/en/pdf/Press_release_resolution_1_CGPM.pdf | | url = http://www.bipm.org/utils/en/pdf/Press_release_resolution_1_CGPM.pdf | ||
| | title = General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram. |
| title = General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram. | ||
| | publisher = ] | | publisher = ] | ||
| | location = Sèvres, France | | location = Sèvres, France | ||
| | date = 23 October 2011 | | date = 23 October 2011 | ||
| | |
| access-date = 25 October 2011 | ||
| | url-status = dead | |||
| | archive-url = https://web.archive.org/web/20120209175127/http://www.bipm.org/utils/en/pdf/Press_release_resolution_1_CGPM.pdf | |||
| The redefinition was further postponed in 2014, pending more accurate measurements of Boltzmann's constant in terms of the current definition, but it is expected to be adopted at the 26th CGPM in late 2018.<ref>{{Cite web | |||
| | archive-date = 9 February 2012 | |||
| }}</ref> A challenge was to avoid degrading the accuracy of measurements close to the triple point. The redefinition was further postponed in 2014, pending more accurate measurements of the Boltzmann constant in terms of the current definition,<ref> | |||
| {{Cite web | |||
| |url=http://www.bipm.org/cc/TGFC/Allowed/Minutes/CODATA_Minutes_14-BIPM-public.pdf | |url=http://www.bipm.org/cc/TGFC/Allowed/Minutes/CODATA_Minutes_14-BIPM-public.pdf | ||
| |title=Report on the Meeting of the CODATA Task Group on Fundamental Constants | |title=Report on the Meeting of the CODATA Task Group on Fundamental Constants | ||
| |date=3–4 November 2014 | |date=3–4 November 2014 | ||
| |place=] | |place=] | ||
| |first=B. |
|first=B. | ||
| |last=Wood | |||
| |page=7 | |page=7 | ||
| |quote= Milton responded to a question about what would happen if ... the CIPM or the CGPM voted not to move forward with the redefinition of the SI. He responded that he felt that by that time the decision to move forward should be seen as a foregone conclusion. | |quote= Milton responded to a question about what would happen if ... the CIPM or the CGPM voted not to move forward with the redefinition of the SI. He responded that he felt that by that time the decision to move forward should be seen as a foregone conclusion. | ||
| |url-status=dead | |||
| }}</ref> | |||
| |archive-url=https://web.archive.org/web/20151013174929/http://www.bipm.org/cc/TGFC/Allowed/Minutes/CODATA_Minutes_14-BIPM-public.pdf | |||
| |archive-date=13 October 2015 | |||
| }}</ref> but was finally adopted at the 26th CGPM in late 2018, with a value of {{math|''k''<sub>B</sub>}} = {{physconst|k|after=.}}<ref name="draft" /><ref name="MEP for kelvin 2019" /><ref name="SI Brochure 9" /><ref name="NIST SI redefinition" /><ref name="CGPM 26 res 1 (2018)" /> | |||
| For scientific purposes, the redefinition's main advantage is in allowing more accurate measurements at very low and very high temperatures, as the techniques used depend on the Boltzmann constant. Independence from any particular substance or measurement is also a philosophical advantage. The kelvin now only depends on the Boltzmann constant and ] (see 2019 SI unit dependencies diagram), allowing the kelvin to be expressed exactly as:<ref name="SI Brochure 9" /> | |||
| From a scientific point of view, this will link temperature to the rest of SI and result in a stable definition that is independent of any particular substance. From a practical point of view, the redefinition will pass unnoticed; water will still freeze at {{convert|273.15|K|abbr=on}}.<ref>{{cite web |url=http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Updating_the_definition_of_the_kelvin2.pdf |title=Updating the definition of the kelvin |publisher=International Bureau for Weights and Measures (]) |accessdate=2010-02-23}}</ref> | |||
| : 1 kelvin = {{Nowrap|{{sfrac|{{val|1.380649|e=-23}}|({{val|6.62607015|e=-34}})({{val|9192631770}})}} {{Math|{{sfrac|{{gaps|]|]}}|]}}}}}} = {{Nowrap|{{sfrac|{{val|13.80649}}|{{val|6.09110229711386655}}}} {{Math|{{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''k''<sub>B</sub>}}}}}}. | |||
| ==Practical uses== | |||
| For practical purposes, the redefinition was unnoticed; enough digits were used for the Boltzmann constant to ensure that 273.16 K has enough ] to contain the uncertainty of water's triple point<ref name="codata2017" /> and water still normally freezes at 0 °C<ref>{{cite web |url=http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Updating_the_definition_of_the_kelvin2.pdf |title=Updating the definition of the kelvin |publisher=] |access-date=2010-02-23 |url-status=dead |archive-url=https://web.archive.org/web/20081123043044/http://www.bipm.org/wg/CCT/TG-SI/Allowed/Documents/Updating_the_definition_of_the_kelvin2.pdf |archive-date=23 November 2008}}</ref> to a high degree of precision. But before the redefinition, the triple point of water was exact and the Boltzmann constant had a measured value of {{val|1.38064903|(51)|e=-23|u=J|up=K}}, with a relative standard uncertainty of {{val|3.7|e=-7}}.<ref name=codata2017> | |||
| ===Colour temperature=== | |||
| {{cite journal | |||
| <!--NOTE TO EDITORS: With regard to UK vs. American spelling, per ], the term originally used in the article by the first major contributor(s) should be retained.]]--> | |||
| |title=The CODATA 2017 values of ''h'', ''e'', ''k'', and ''N''<sub>A</sub> for the revision of the SI | |||
| {{main|Colour temperature}} | |||
| |collaboration=Committee on Data for Science and Technology (CODATA) Task Group on Fundamental Constants | |||
| |first1=D B |last1=Newell |first2=F |last2=Cabiati |first3=J |last3=Fischer |first4=K |last4=Fujii | |||
| |first5=S G |last5=Karshenboim |first6=H S |last6=Margolis |first7=E |last7=de Mirandés | |||
| |first8=P J |last8=Mohr |first9=F |last9=Nez |first10=K |last10=Pachucki |first11=T J |last11=Quinn | |||
| |first12=B N |last12=Taylor |first13=M |last13=Wang |first14=B M |last14=Wood |first15=Z |last15=Zhang | |||
| |journal=Metrologia |volume=55 |issue=1 |pages=L13–L16 | |||
| |date=29 January 2018 | |||
| |doi=10.1088/1681-7575/aa950a |bibcode=2018Metro..55L..13N | |||
| |doi-access=free |bibcode-access=free | |||
| }}</ref> Afterward, the Boltzmann constant is exact and the uncertainty is transferred to the triple point of water, which is now {{val|273.1600|(1)|u=K}}.{{efn|name=uncertainty|The absolute uncertainty can be calculated as {{math|273.16 × {{val|3.7|e=-7|u=K}}}}, which can be rounded to {{val|0.10|u=mK}} for all practical purposes.<ref>{{cite journal |last1=Fischer |first1=J |last2=Fellmuth |first2=B |last3=Gaiser |first3=C |last4=Zandt |first4=T |last5=Pitre |first5=L |last6=Sparasci |first6=F |last7=Plimmer |first7=M D |last8=de Podesta |first8=M |last9=Underwood |first9=R |last10=Sutton |first10=G |last11=Machin |first11=G |last12=Gavioso |first12=R M |last13=Madonna Ripa |first13=D |last14=Steur |first14=P P M |last15=Qu |first15=J |last16=Feng |first16=X J |last17=Zhang |first17=J |last18=Moldover |first18=M R |last19=Benz |first19=S P |last20=White |first20=D R |last21=Gianfrani |first21=L |last22=Castrillo |first22=A |last23=Moretti |first23=L |last24=Darquié |first24=B |last25=Moufarej |first25=E |last26=Daussy |first26=C |last27=Briaudeau |first27=S |last28=Kozlova |first28=O |last29=Risegari |first29=L |last30=Segovia |first30=J J |last31=Martín |first31=M C |last32=del Campo |first32=D |title=The Boltzmann project |journal=Metrologia |date=1 April 2018 |volume=55 |issue=2 |pages=R1–R20 |doi=10.1088/1681-7575/aaa790 |pmid=31080297|pmc=6508687|bibcode=2018Metro..55R...1F }}</ref>}} | |||
| The new definition officially came into force on 20 May 2019, the 144th anniversary of the ].<ref name="CGPM 26 res 1 (2018)">{{cite web |title=Resolution 1 of the 26th CGPM (2018) |url=https://www.bipm.org/en/committees/cg/cgpm/26-2018/resolution-1 |publisher=BIPM |access-date=21 February 2022}}</ref><ref name="MEP for kelvin 2019" /><ref name="SI Brochure 9" /><ref name="NIST SI redefinition" /> | |||
| == Practical uses == | |||
| ] (right) of various light sources (left)]] | |||
| === Colour temperature === | |||
| {{see also|Stefan–Boltzmann constant}} | {{see also|Stefan–Boltzmann constant}} | ||
| The kelvin is often used |
The kelvin is often used as a measure of the ] of light sources. Colour temperature is based upon the principle that a ] radiator emits light with a frequency distribution characteristic of its temperature. Black bodies at temperatures below about {{val|4000|u=K}} appear reddish, whereas those above about {{val|7500|u=K}} appear bluish. Colour temperature is important in the fields of image projection and photography, where a colour temperature of approximately {{val|5600|u=K}} is required to match "daylight" film emulsions. | ||
| In ], the ] of stars and their place on the ] are based, in part, upon their surface temperature, known as ]. The photosphere of the ], for instance, has an effective temperature of {{val|5772|u=K}} as adopted by IAU 2015 Resolution B3. | |||
| Digital cameras and photographic software often use colour temperature in K in edit and setup menus. The simple guide is that |
Digital cameras and photographic software often use colour temperature in K in edit and setup menus. The simple guide is that higher colour temperature produces an image with enhanced white and blue hues. The reduction in colour temperature produces an image more dominated by reddish, ]. | ||
| ===Kelvin as a |
=== Kelvin as a unit of noise temperature === | ||
| {{main|Noise figure}} | {{main|Noise figure}} | ||
| For ], the kelvin is used as an indicator of how ] a circuit is in relation to an ultimate ], i.e. the ]. The ] of ] (which produces an associated ] when combined with ]) is a type of thermal noise derived from the ] and can be used to determine the noise temperature of a ] using the ]. | |||
| == Derived units and SI multiples == | |||
| == Unicode character == | |||
| {{main|Orders of magnitude (temperature)}} | |||
| The symbol is encoded in ] at codepoint {{unichar|212A|kelvin sign}}. However, this is a ] provided for compatibility with legacy encodings. The Unicode standard recommends using {{unichar|004B|latin capital letter k}} instead; that is, a normal capital K. "Three letterlike symbols have been given canonical equivalence to regular letters: {{unichar|2126|ohm sign}}, {{unichar|212A|kelvin sign}}, and {{unichar|212B|angstrom sign}}. In all three instances, the regular letter should be used."<ref>{{cite book|title=The Unicode Standard, Version 8.0|date=August 2015|publisher=The Unicode Consortium|location=Mountain View, CA, USA|isbn=978-1-936213-10-8 |section=22.2|url=http://www.unicode.org/versions/Unicode8.0.0/ch22.pdf|accessdate=6 September 2015}}</ref> | |||
| The only ] derived from the kelvin is the degree Celsius. Like other SI units, the kelvin can also be modified by adding a ] that multiplies it by a ]: | |||
| {{SI multiples | |||
| | unit=kelvin | |||
| | symbol=K | |||
| }} | |||
| == |
== Orthography == | ||
| According to SI convention, the kelvin is never referred to nor written as a ]. The word "kelvin" is not capitalized when used as a unit. It may be in plural form as appropriate (for example, "it is 283 kelvins outside", as for "it is 50 degrees Fahrenheit" and "10 degrees Celsius").<ref name="plurals">{{citation |work=NIST SP 811 |title=NIST Guide to the SI {{!}} Chapter 9: Rules and Style Conventions for Spelling Unit Names |date=28 January 2016 |url=https://www.nist.gov/pml/special-publication-811/nist-guide-si-chapter-9-rules-and-style-conventions-spelling-unit-names#97 |quote=A derived unit is usually singular in English, for example, the value 3 m<sup>2</sup>·K/W is usually spelled out as 'three square meter kelvin per watt', and the value 3 C·m<sup>2</sup>/V is usually spelled out as 'three coulomb meter squared per volt'. However, a 'single' unit may be plural; for example, the value 5 kPa is spelled out as 'five kilopascals', although 'five kilopascal' is acceptable. If in such a single-unit case the number is less than one, the unit is always singular when spelled out; for example, 0.5 kPa is spelled out as 'five-tenths kilopascal'. }}</ref><ref name="nist intro"/><ref>{{Cite web |title=Definition of KELVIN |url=https://www.merriam-webster.com/dictionary/kelvin |access-date=2023-08-21 |website=www.merriam-webster.com |language=en}}</ref><ref>{{Cite book |url=https://translation-council-support-group.web.cern.ch/sites/default/files/styles/large/CERN%20TM%20English%20language%20style%20guide.pdf |title=CERN English Language Style Guide |publisher=] |year=2022 |pages=64}}</ref> The unit's symbol K is a capital letter,<ref name="CGPM 13 res 3 (1967)">{{cite web |title=Resolution 3 of the 13th CGPM (1967) |url=https://www.bipm.org/en/committees/cg/cgpm/13-1967/resolution-3 |publisher=BIPM |access-date=2022-02-21}}</ref> per the SI convention to capitalize symbols of units derived from the name of a person.<ref>{{Cite journal |date=2010-01-13 |title=Writing with SI (Metric System) Units |url=https://www.nist.gov/pml/owm/writing-si-metric-system-units |journal=NIST |language=en}}</ref> It is common convention to capitalize Kelvin when referring to Lord Kelvin<ref name="nist intro"/> or the Kelvin scale.<ref>{{cite book |last1=Brady |first1=James E. |last2=Senese |first2=Fred |title=Chemistry, Student Study Guide: The Study of Matter and Its Changes |date=28 January 2008 |publisher=John Wiley & Sons |isbn=978-0-470-18464-6 |page=15 |url=https://books.google.com/books?id=zS1EX-e7kPQC&pg=PA15 |language=en}}</ref> | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| The unit symbol K is encoded in ] at code point {{unichar|212A|kelvin sign}}. However, this is a ] provided for compatibility with legacy encodings. The Unicode standard recommends using {{unichar|004B|latin capital letter ]}} instead; that is, a normal capital ]. "Three letterlike symbols have been given canonical equivalence to regular letters: {{unichar|2126|ohm sign}}, {{unichar|212A|kelvin sign}}, and {{unichar|212B|angstrom sign}}. In all three instances, the regular letter should be used."<ref>{{cite book|title=The Unicode Standard, Version 8.0|date=August 2015|publisher=The Unicode Consortium|location=Mountain View, CA, USA|isbn=978-1-936213-10-8|section=22.2|url=https://www.unicode.org/versions/Unicode8.0.0/ch22.pdf|access-date=6 September 2015|url-status=live|archive-url=https://web.archive.org/web/20161206230132/http://www.unicode.org/versions/Unicode8.0.0/ch22.pdf|archive-date=6 December 2016}}</ref> | |||
| == Notes and references== | |||
| {{Reflist|30em}} | |||
| == |
== See also == | ||
| {{Portal|Energy}} | |||
| * ] | |||
| * ] | |||
| * ] – product of the Boltzmann constant and temperature | |||
| * ] | |||
| * ] | |||
| == Notes == | |||
| {{notelist}} | |||
| == References == | |||
| {{Reflist|refs= | |||
| <ref name="SI Brochure 9">{{cite web |title=SI Brochure: The International System of Units (SI) – 9th edition (updated in 2022) |url=https://www.bipm.org/documents/20126/41483022/SI-Brochure-9-EN.pdf/2d2b50bf-f2b4-9661-f402-5f9d66e4b507 |publisher=BIPM |access-date=7 September 2022}}</ref> | |||
| <ref name="BIPM web page for kelvin">{{cite web |title=SI base unit: kelvin (K) |url=https://www.bipm.org/en/si-base-units/kelvin |publisher=BIPM |access-date=5 March 2022}}</ref> | |||
| <ref name="NIST SI redefinition">{{cite journal |title=A Turning Point for Humanity: Redefining the World's Measurement System |url=https://www.nist.gov/si-redefinition/turning-point-humanity-redefining-worlds-measurement-system |journal=NIST |date=12 May 2018 |access-date=21 February 2022}}</ref> | |||
| <ref name="MEP for kelvin 2019">{{cite web |last=BIPM |date=20 May 2019 |title=Mise en pratique for the definition of the kelvin in the SI |url=https://www.bipm.org/documents/20126/41489682/SI-App2-kelvin.pdf/cd36cb68-3f00-05fd-339e-452df0b6215e?version=1.5&t=1637237805352&download=false |access-date=18 February 2022 |website=BIPM.org}}</ref> | |||
| <ref name="nist intro">{{cite web |title=Kelvin: Introduction |url=https://www.nist.gov/si-redefinition/kelvin-introduction |website=NIST |access-date=2 September 2022 |language=en |date=14 May 2018}}</ref> | |||
| <ref name="NIST HB44 Appendix C">{{cite web |title=Handbook 44 – 2022 – Appendix C – General Tables of Units of Measurement |url=https://www.nist.gov/system/files/documents/2021/11/30/2022-HB44-Section-Appendix-C.pdf |website=nist.gov |publisher=NIST |access-date=21 February 2022}}</ref> | |||
| <ref name="Busting Myths about the Metric System">{{cite journal |author-last=Benham |author-first=Elizabeth |title=Busting Myths about the Metric System |url=https://www.nist.gov/blogs/taking-measure/busting-myths-about-metric-system |journal=NIST |date=6 October 2020 |publisher=Taking Measure (official blog of the NIST) |access-date=21 February 2022}}</ref> | |||
| <ref name="Dalton_1801_1">{{Cite journal |author-first=John |author-last=Dalton |date=1801 |url=https://books.google.com/books?id=3qdJAAAAYAAJ&pg=PA595 |title=Essay II. On the force of steam or vapour from water and various other liquids, both in vacuum and in air |journal=Memoirs of the Literary and Philosophical Society of Manchester |volume=5 part 2 |pages=550–574}}</ref> | |||
| <ref name="Dalton_1801_2">{{Cite journal |author-first=John |author-last=Dalton |date=1801 |url=https://books.google.com/books?id=3qdJAAAAYAAJ&pg=PA595 |title=Essay IV. On the expansion of elastic fluids by heat |journal=Memoirs of the Literary and Philosophical Society of Manchester |volume=5 part 2 |pages=595–602}}</ref> | |||
| <ref name="Gay-Lussac_1802">{{citation |author-last=Gay-Lussac |author-first=Joseph Louis |author-link=Joseph Louis Gay-Lussac |date=1802 |title=Recherches sur la dilatation des gaz et des vapeurs |journal=Annales de Chimie |volume=XLIII |page=137}}. </ref> | |||
| <ref name="Westphal_1952">{{cite book |title=Physikalisches Wörterbuch |language=de |trans-title= |chapter=Nox, Dunkelleuchtdichte, Skot |author-first=Wilhelm Heinrich |author-last=Westphal |editor-first1=Wilhelm H. |editor-last1=Westphal |author-link=Wilhelm Heinrich Westphal |date=1952 |edition=1 |publisher=] |publication-place=Berlin / Göttingen / Heidelberg, Germany |isbn=978-3-662-12707-0 |doi=10.1007/978-3-662-12706-3 |pages=125, 271, 389 |chapter-url=https://books.google.com/books?id=QaCFBwAAQBAJ&pg=RA1-PA125 |access-date=2023-03-16 |quote-pages=271, 389 |quote=Dunkelleuchtdichte. Unter Zugrundelegung dieser Empfindlichkeitskurve hat man 1940 in Deutschland die Dunkelleuchtdichte mit der Einheit ] (sk) so festgesetzt, daß bei einem Licht der Farbtemperatur 2360 {{not a typo|°]}} 1 sk = 10<sup>−3</sup> asb gilt. 1948 ist von der ] (IBK) die Bezugstemperatur auf 2046 {{not a typo|°K}}, die Erstarrungstemperatur des ]s, festgesetzt worden. Die Bezeichnung Skot wurde von der IBK nicht übernommen, dafür soll "skotopisches Stilb" gesagt werden. Als höchstzulässiger Grenzwert für die Dunkelleuchtdichte ist in Deutschland 10 Skot festgesetzt worden, um eine Verwendung der Dunkelleuchtdichte im Gebiet des gemischten ]- und ]s zu vermeiden, da in diesem Bereich die photometrischen Maßgrößen wegen der allmählich gleitenden Augenempfindlichkeitskurve ihren Sinn verlieren. Skot, abgek sk, Einheit für die Dunkelleuchtdichte, welche für zahlenmäßige Angaben und zum Anschluß der Dunkelleuchtdichte an die normale Leuchtdichte 1940 von der {{ill|German Lighting Society|de|Deutsche Lichttechnische Gesellschaft|lt=Deutschen Lichttechnischen Gesellschaft}} geschaffen wurde. Für diesen Anschluß wurde die Strahlung des ]s bei ''T'' = 2360 {{not a typo|°K}}, d.h. eine Strahlung der Farbtemperatur ''T''<sub>1</sub> = 2360 {{not a typo|°K}} vereinbart. Eine Lichtquelle strahlt mit der Dunkelleuchtdichte 1 sk, wenn sie photometrisch gleich einer Strahlung der Farbtemperatur ''T''<sub>2</sub> = 2360 {{not a typo|°K}} und der Leuchtdichte von 10<sup>−3</sup> asb (Apostilb) ist. Bei der Farbtemperatur ''T''<sub>1</sub> = 2360 {{not a typo|°K}} gilt also die Relation: 1 sk = 10<sup>−3</sup> asb = 10<sup>−7</sup>/π sb. |trans-quote=}}</ref> | |||
| }} | |||
| == Bibliography == | |||
| * {{cite web |author=Bureau International des Poids et Mesures |title=The International System of Units (SI) Brochure |version=9th Edition |publisher=International Committee for Weights and Measures |url=https://www.bipm.org/documents/20126/41483022/SI-Brochure-9-EN.pdf |date=2019 |access-date=2022-04-28}} | |||
| * {{cite book |last1=Thomson |first1=William (Lord Kelvin) |title=Mathematical and physical papers: Volume I |date=1882 |publisher=Cambridge University Press |url=https://archive.org/details/mathematicalphys01kelv}} | |||
| == External links == | |||
| {{wiktionary}} | {{wiktionary}} | ||
| * {{cite web |author-last=Thomson |author-first=William |title=On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations |url=https://zapatopi.net/kelvin/papers/on_an_absolute_thermometric_scale.html |website=zapatopi.net |publisher=Philosophical Magazine|date=October 1848 |access-date=21 February 2022|archive-url=https://web.archive.org/web/20080201095927/http://zapatopi.net/kelvin/papers/on_an_absolute_thermometric_scale.html |archive-date=1 February 2008}} | |||
| * {{cite web |author-last=Thomson |author-first=William |title=On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule's equivalent of a Thermal Unit, and M. Regnault's Observations on Steam |url=https://zapatopi.net/kelvin/papers/on_the_dynamical_theory_of_heat.html |website=zapatopi.net |publisher= | |||
| Transactions of the Royal Society of Edinburgh|date=March 1851|access-date=5 May 2024}} | |||
| *{{cite journal |author=Bureau International des Poids et Mesures |title=The International System of Units (SI) Brochure |version=8th Edition |publisher=International Committee for Weights and Measures |url=http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf |year=2006 |accessdate=2008-02-06}} | |||
| * | |||
| {{Scales of temperature}} | {{Scales of temperature}} | ||
| {{SI units}} | {{SI units}} | ||
| {{ |
{{CGS units}} | ||
| {{Use dmy dates|date=August 2011}} | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 07:57, 25 December 2024
SI unit of temperature This article is about the unit of temperature. For the person, see Lord Kelvin. For other uses, see Kelvin (disambiguation).
| kelvin | |
|---|---|
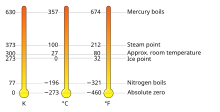 Equivalent temperatures in kelvin (K), Celsius (°C), and Fahrenheit (°F) Equivalent temperatures in kelvin (K), Celsius (°C), and Fahrenheit (°F) | |
| General information | |
| Unit system | SI |
| Unit of | temperature |
| Symbol | K |
| Named after | William Thomson, 1st Baron Kelvin |
| 2019 definition | kB ≝ 1.380649×10 J/K |
| Conversions | |
| x K in ... | ... corresponds to ... |
| SI derived units | (x − 273.15) °C |
| Imperial/US units | (1.8x − 459.67) °F |
| Imperial/US absolute scale | 1.8x °Ra |
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15.
The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Boltzmann constant to exactly 1.380649×10 joules per kelvin; every 1 K change of thermodynamic temperature corresponds to a thermal energy change of exactly 1.380649×10 J.
History
See also: Thermodynamic temperature § HistoryPrecursors

During the 18th century, multiple temperature scales were developed, notably Fahrenheit and centigrade (later Celsius). These scales predated much of the modern science of thermodynamics, including atomic theory and the kinetic theory of gases which underpin the concept of absolute zero. Instead, they chose defining points within the range of human experience that could be reproduced easily and with reasonable accuracy, but lacked any deep significance in thermal physics. In the case of the Celsius scale (and the long since defunct Newton scale and Réaumur scale) the melting point of ice served as such a starting point, with Celsius being defined (from the 1740s to the 1940s) by calibrating a thermometer such that:
- Water's freezing point is 0 °C.
- Water's boiling point is 100 °C.
This definition assumes pure water at a specific pressure chosen to approximate the natural air pressure at sea level. Thus, an increment of 1 °C equals 1/100 of the temperature difference between the melting and boiling points. The same temperature interval was later used for the Kelvin scale.
Charles's law
From 1787 to 1802, it was determined by Jacques Charles (unpublished), John Dalton, and Joseph Louis Gay-Lussac that, at constant pressure, ideal gases expanded or contracted their volume linearly (Charles's law) by about 1/273 parts per degree Celsius of temperature's change up or down, between 0 °C and 100 °C. Extrapolation of this law suggested that a gas cooled to about −273 °C would occupy zero volume.
Lord Kelvin

First absolute scale
In 1848, William Thomson, who was later ennobled as Lord Kelvin, published a paper On an Absolute Thermometric Scale. The scale proposed in the paper turned out to be unsatisfactory, but the principles and formulas upon which the scale was based were correct. For example, in a footnote, Thomson derived the value of −273 °C for absolute zero by calculating the negative reciprocal of 0.00366—the coefficient of thermal expansion of an ideal gas per degree Celsius relative to the ice point. This derived value agrees with the currently accepted value of −273.15 °C, allowing for the precision and uncertainty involved in the calculation.
The scale was designed on the principle that "a unit of heat descending from a body A at the temperature T° of this scale, to a body B at the temperature (T − 1)°, would give out the same mechanical effect, whatever be the number T." Specifically, Thomson expressed the amount of work necessary to produce a unit of heat (the thermal efficiency) as , where is the temperature in Celsius, is the coefficient of thermal expansion, and was "Carnot's function", a substance-independent quantity depending on temperature, motivated by an obsolete version of Carnot's theorem. The scale is derived by finding a change of variables of temperature such that is proportional to .

When Thomson published his paper in 1848, he only considered Regnault's experimental measurements of . That same year, James Prescott Joule suggested to Thomson that the true formula for Carnot's function was where is "the mechanical equivalent of a unit of heat", now referred to as the specific heat capacity of water, approximately 771.8 foot-pounds force per degree Fahrenheit per pound (4,153 J/K/kg). Thomson was initially skeptical of the deviations of Joule's formula from experiment, stating "I think it will be generally admitted that there can be no such inaccuracy in Regnault's part of the data, and there remains only the uncertainty regarding the density of saturated steam". Thomson referred to the correctness of Joule's formula as "Mayer's hypothesis", on account of it having been first assumed by Mayer. Thomson arranged numerous experiments in coordination with Joule, eventually concluding by 1854 that Joule's formula was correct and the effect of temperature on the density of saturated steam accounted for all discrepancies with Regnault's data. Therefore, in terms of the modern Kelvin scale , the first scale could be expressed as follows: The parameters of the scale were arbitrarily chosen to coincide with the Celsius scale at 0° and 100 °C or 273 and 373 K (the melting and boiling points of water). On this scale, an increase of approximately 222 degrees corresponds to a doubling of Kelvin temperature, regardless of the starting temperature, and "infinite cold" (absolute zero) has a numerical value of negative infinity.
Modern absolute scale
Thomson understood that with Joule's proposed formula for , the relationship between work and heat for a perfect thermodynamic engine was simply the constant . In 1854, Thomson and Joule thus formulated a second absolute scale that was more practical and convenient, agreeing with air thermometers for most purposes. Specifically, "the numerical measure of temperature shall be simply the mechanical equivalent of the thermal unit divided by Carnot's function."
To explain this definition, consider a reversible Carnot cycle engine, where is the amount of heat energy transferred into the system, is the heat leaving the system, is the work done by the system (), is the temperature of the hot reservoir in Celsius, and is the temperature of the cold reservoir in Celsius. The Carnot function is defined as , and the absolute temperature as . One finds the relationship . By supposing , one obtains the general principle of an absolute thermodynamic temperature scale for the Carnot engine, . The definition can be shown to correspond to the thermometric temperature of the ideal gas laws.
This definition by itself is not sufficient. Thomson specified that the scale should have two properties:
- The absolute values of two temperatures are to one another in the proportion of the heat taken in to the heat rejected in a perfect thermodynamic engine working with a source and refrigerator at the higher and lower of the temperatures respectively.
- The difference of temperatures between the freezing- and boiling-points of water under standard atmospheric pressure shall be called 100 degrees. (The same increment as the Celsius scale) Thomson's best estimates at the time were that the temperature of freezing water was 273.7 K and the temperature of boiling water was 373.7 K.
These two properties would be featured in all future versions of the Kelvin scale, although it was not yet known by that name. In the early decades of the 20th century, the Kelvin scale was often called the "absolute Celsius" scale, indicating Celsius degrees counted from absolute zero rather than the freezing point of water, and using the same symbol for regular Celsius degrees, °C.
Triple point standard

In 1873, William Thomson's older brother James coined the term triple point to describe the combination of temperature and pressure at which the solid, liquid, and gas phases of a substance were capable of coexisting in thermodynamic equilibrium. While any two phases could coexist along a range of temperature-pressure combinations (e.g. the boiling point of water can be affected quite dramatically by raising or lowering the pressure), the triple point condition for a given substance can occur only at a single pressure and only at a single temperature. By the 1940s, the triple point of water had been experimentally measured to be about 0.6% of standard atmospheric pressure and very close to 0.01 °C per the historical definition of Celsius then in use.
In 1948, the Celsius scale was recalibrated by assigning the triple point temperature of water the value of 0.01 °C exactly and allowing the melting point at standard atmospheric pressure to have an empirically determined value (and the actual melting point at ambient pressure to have a fluctuating value) close to 0 °C. This was justified on the grounds that the triple point was judged to give a more accurately reproducible reference temperature than the melting point. The triple point could be measured with ±0.0001 °C accuracy, while the melting point just to ±0.001 °C.
In 1954, with absolute zero having been experimentally determined to be about −273.15 °C per the definition of °C then in use, Resolution 3 of the 10th General Conference on Weights and Measures (CGPM) introduced a new internationally standardized Kelvin scale which defined the triple point as exactly 273.15 + 0.01 = 273.16 degrees Kelvin.
In 1967/1968, Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature "kelvin", symbol K, replacing "degree Kelvin", symbol °K. The 13th CGPM also held in Resolution 4 that "The kelvin, unit of thermodynamic temperature, is equal to the fraction 1/273.16 of the thermodynamic temperature of the triple point of water."
After the 1983 redefinition of the metre, this left the kelvin, the second, and the kilogram as the only SI units not defined with reference to any other unit.
In 2005, noting that the triple point could be influenced by the isotopic ratio of the hydrogen and oxygen making up a water sample and that this was "now one of the major sources of the observed variability between different realizations of the water triple point", the International Committee for Weights and Measures (CIPM), a committee of the CGPM, affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the kelvin would refer to water having the isotopic composition specified for Vienna Standard Mean Ocean Water.
2019 redefinition
Main article: 2019 revision of the SI
In 2005, the CIPM began a programme to redefine the kelvin (along with other SI base units) using a more experimentally rigorous method. In particular, the committee proposed redefining the kelvin such that the Boltzmann constant (kB) would take the exact value 1.3806505×10 J/K. The committee hoped the program would be completed in time for its adoption by the CGPM at its 2011 meeting, but at the 2011 meeting the decision was postponed to the 2014 meeting when it would be considered part of a larger program. A challenge was to avoid degrading the accuracy of measurements close to the triple point. The redefinition was further postponed in 2014, pending more accurate measurements of the Boltzmann constant in terms of the current definition, but was finally adopted at the 26th CGPM in late 2018, with a value of kB = 1.380649×10 J⋅K.
For scientific purposes, the redefinition's main advantage is in allowing more accurate measurements at very low and very high temperatures, as the techniques used depend on the Boltzmann constant. Independence from any particular substance or measurement is also a philosophical advantage. The kelvin now only depends on the Boltzmann constant and universal constants (see 2019 SI unit dependencies diagram), allowing the kelvin to be expressed exactly as:
- 1 kelvin = 1.380649×10/(6.62607015×10)(9192631770) hΔνCs/kB = 13.80649/6.09110229711386655 hΔνCs/kB.
For practical purposes, the redefinition was unnoticed; enough digits were used for the Boltzmann constant to ensure that 273.16 K has enough significant digits to contain the uncertainty of water's triple point and water still normally freezes at 0 °C to a high degree of precision. But before the redefinition, the triple point of water was exact and the Boltzmann constant had a measured value of 1.38064903(51)×10 J/K, with a relative standard uncertainty of 3.7×10. Afterward, the Boltzmann constant is exact and the uncertainty is transferred to the triple point of water, which is now 273.1600(1) K.
The new definition officially came into force on 20 May 2019, the 144th anniversary of the Metre Convention.
Practical uses
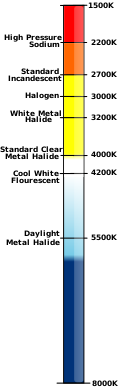
Colour temperature
See also: Stefan–Boltzmann constantThe kelvin is often used as a measure of the colour temperature of light sources. Colour temperature is based upon the principle that a black body radiator emits light with a frequency distribution characteristic of its temperature. Black bodies at temperatures below about 4000 K appear reddish, whereas those above about 7500 K appear bluish. Colour temperature is important in the fields of image projection and photography, where a colour temperature of approximately 5600 K is required to match "daylight" film emulsions.
In astronomy, the stellar classification of stars and their place on the Hertzsprung–Russell diagram are based, in part, upon their surface temperature, known as effective temperature. The photosphere of the Sun, for instance, has an effective temperature of 5772 K as adopted by IAU 2015 Resolution B3.
Digital cameras and photographic software often use colour temperature in K in edit and setup menus. The simple guide is that higher colour temperature produces an image with enhanced white and blue hues. The reduction in colour temperature produces an image more dominated by reddish, "warmer" colours.
Kelvin as a unit of noise temperature
Main article: Noise figureFor electronics, the kelvin is used as an indicator of how noisy a circuit is in relation to an ultimate noise floor, i.e. the noise temperature. The Johnson–Nyquist noise of resistors (which produces an associated kTC noise when combined with capacitors) is a type of thermal noise derived from the Boltzmann constant and can be used to determine the noise temperature of a circuit using the Friis formulas for noise.
Derived units and SI multiples
Main article: Orders of magnitude (temperature)The only SI derived unit with a special name derived from the kelvin is the degree Celsius. Like other SI units, the kelvin can also be modified by adding a metric prefix that multiplies it by a power of 10:
| Submultiples | Multiples | ||||
|---|---|---|---|---|---|
| Value | SI symbol | Name | Value | SI symbol | Name |
| 10 K | dK | decikelvin | 10 K | daK | decakelvin |
| 10 K | cK | centikelvin | 10 K | hK | hectokelvin |
| 10 K | mK | millikelvin | 10 K | kK | kilokelvin |
| 10 K | μK | microkelvin | 10 K | MK | megakelvin |
| 10 K | nK | nanokelvin | 10 K | GK | gigakelvin |
| 10 K | pK | picokelvin | 10 K | TK | terakelvin |
| 10 K | fK | femtokelvin | 10 K | PK | petakelvin |
| 10 K | aK | attokelvin | 10 K | EK | exakelvin |
| 10 K | zK | zeptokelvin | 10 K | ZK | zettakelvin |
| 10 K | yK | yoctokelvin | 10 K | YK | yottakelvin |
| 10 K | rK | rontokelvin | 10 K | RK | ronnakelvin |
| 10 K | qK | quectokelvin | 10 K | QK | quettakelvin |
Orthography
According to SI convention, the kelvin is never referred to nor written as a degree. The word "kelvin" is not capitalized when used as a unit. It may be in plural form as appropriate (for example, "it is 283 kelvins outside", as for "it is 50 degrees Fahrenheit" and "10 degrees Celsius"). The unit's symbol K is a capital letter, per the SI convention to capitalize symbols of units derived from the name of a person. It is common convention to capitalize Kelvin when referring to Lord Kelvin or the Kelvin scale.
The unit symbol K is encoded in Unicode at code point U+212A K KELVIN SIGN. However, this is a compatibility character provided for compatibility with legacy encodings. The Unicode standard recommends using U+004B K LATIN CAPITAL LETTER K instead; that is, a normal capital K. "Three letterlike symbols have been given canonical equivalence to regular letters: U+2126 Ω OHM SIGN, U+212A K KELVIN SIGN, and U+212B Å ANGSTROM SIGN. In all three instances, the regular letter should be used."
See also
- Comparison of temperature scales
- International Temperature Scale of 1990
- kT (energy) – product of the Boltzmann constant and temperature
- Negative temperature
- Outline of metrology and measurement
Notes
- The absolute uncertainty can be calculated as 273.16 × 3.7×10 K, which can be rounded to 0.10 mK for all practical purposes.
References
- ^ BIPM (2019-05-20). "Mise en pratique for the definition of the kelvin in the SI". BIPM.org. Retrieved 2022-02-18.
- ^ "SI Brochure: The International System of Units (SI) – 9th edition (updated in 2022)". BIPM. Retrieved 2022-09-07.
- "SI base unit: kelvin (K)". BIPM. Retrieved 2022-03-05.
- ^ "A Turning Point for Humanity: Redefining the World's Measurement System". NIST. 2018-05-12. Retrieved 2022-02-21.
- ^ "Kelvin: Introduction". NIST. 2018-05-14. Retrieved 2022-09-02.
- ^ Encyclopaedia Britannica editions from the 1920s and 1950s, the article "Planets".
- Benham, Elizabeth (2020-10-06). "Busting Myths about the Metric System". NIST. Taking Measure (official blog of the NIST). Retrieved 2022-02-21.
- "Handbook 44 – 2022 – Appendix C – General Tables of Units of Measurement" (PDF). nist.gov. NIST. Retrieved 2022-02-21.
- "Kelvin: History". NIST. 2018-05-14. Retrieved 2022-02-21.
- Dalton, John (1801). "Essay II. On the force of steam or vapour from water and various other liquids, both in vacuum and in air". Memoirs of the Literary and Philosophical Society of Manchester. 5 part 2: 550–574.
- Dalton, John (1801). "Essay IV. On the expansion of elastic fluids by heat". Memoirs of the Literary and Philosophical Society of Manchester. 5 part 2: 595–602.
- Gay-Lussac, Joseph Louis (1802), "Recherches sur la dilatation des gaz et des vapeurs", Annales de Chimie, XLIII: 137. English translation (extract).
- Thomson 1882, pp. 100–106.
- ^ Magie, William Francis (1935). A Source Book In Physics. p. 237.
- Thomson 1882, p. 104: "If we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/·366, if ·366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low."
- Thomson 1882, p. 104.
- Thomson 1882, p. 187.
- ^ Thomson 1882, p. 106.
- Thomson 1882, p. 193.
- Thomson 1882, p. 212.
- Thomson 1882, p. 186.
- Thomson 1882, p. 192.
- Thomson 1882, pp. 214–215.
- Thomson 1882, p. 213.
- Thomson 1882, p. 388.
- Thomson 1882, p. 105: "The arbitrary points which coincide on the two scales are 0° and 100°"
- Saslow, WM (2020-01-07). "A History of Thermodynamics: The Missing Manual". Entropy. 22 (1). eqn. (36). Bibcode:2020Entrp..22...77S. doi:10.3390/e22010077. PMC 7516509. PMID 33285852.
- Thomson 1882, p. 190, formula (7).
- Thomson 1882, pp. 106, 232–236.
- Thomson 1882, p. 234.
- Wang, Lin-Shu (2020). A treatise of heat and energy. Cham: Springer. p. 77. ISBN 9783030057466.
- Thomson 1882, p. 235.
- Thomson 1882, p. 236.
- Thomson, James (1873). "A quantitative investigation of certain relations between the gaseous, the liquid, and the solid states of water-substance". Proceedings of the Royal Society of London. 22: 28. Bibcode:1873RSPS...22...27T. ISSN 0370-1662.
and consequently that the three curves would meet or cross each other in one point, which I have called the triple point.
- ^ Swinton, F. L. (September 1967). "The triplet point of water". Journal of Chemical Education. 44 (9): 541. Bibcode:1967JChEd..44..541S. doi:10.1021/ed044p541. ISSN 0021-9584.
- "Resolution 3 of the 9th CGPM (1948)". BIPM. Retrieved 2022-02-21.
- "Resolution 3 of the 10th CGPM (1954)". BIPM. Retrieved 2022-02-21.
- "Resolution 3: Definition of the thermodynamic temperature scale". Resolutions of the 10th CGPM. Bureau International des Poids et Mesures. 1954. Archived from the original on 2007-06-23. Retrieved 2008-02-06.
- ^ "Resolution 3 of the 13th CGPM (1967)". BIPM. Retrieved 2022-02-21.
- "Resolution 3: SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. Archived from the original on 2007-04-21. Retrieved 2008-02-06.
- Westphal, Wilhelm Heinrich (1952). "Nox, Dunkelleuchtdichte, Skot". In Westphal, Wilhelm H. (ed.). Physikalisches Wörterbuch (in German) (1 ed.). Berlin / Göttingen / Heidelberg, Germany: Springer-Verlag OHG. pp. 125, 271, 389. doi:10.1007/978-3-662-12706-3. ISBN 978-3-662-12707-0. Retrieved 2023-03-16. pp. 271, 389:
Dunkelleuchtdichte. Unter Zugrundelegung dieser Empfindlichkeitskurve hat man 1940 in Deutschland die Dunkelleuchtdichte mit der Einheit Skot (sk) so festgesetzt, daß bei einem Licht der Farbtemperatur 2360 °K 1 sk = 10 asb gilt. 1948 ist von der Internationalen Beleuchtungskommission (IBK) die Bezugstemperatur auf 2046 °K, die Erstarrungstemperatur des Platins, festgesetzt worden. Die Bezeichnung Skot wurde von der IBK nicht übernommen, dafür soll "skotopisches Stilb" gesagt werden. Als höchstzulässiger Grenzwert für die Dunkelleuchtdichte ist in Deutschland 10 Skot festgesetzt worden, um eine Verwendung der Dunkelleuchtdichte im Gebiet des gemischten Zapfen- und Stäbchensehens zu vermeiden, da in diesem Bereich die photometrischen Maßgrößen wegen der allmählich gleitenden Augenempfindlichkeitskurve ihren Sinn verlieren. Skot, abgek sk, Einheit für die Dunkelleuchtdichte, welche für zahlenmäßige Angaben und zum Anschluß der Dunkelleuchtdichte an die normale Leuchtdichte 1940 von der Deutschen Lichttechnischen Gesellschaft [de] geschaffen wurde. Für diesen Anschluß wurde die Strahlung des schwarzen Körpers bei T = 2360 °K, d.h. eine Strahlung der Farbtemperatur T1 = 2360 °K vereinbart. Eine Lichtquelle strahlt mit der Dunkelleuchtdichte 1 sk, wenn sie photometrisch gleich einer Strahlung der Farbtemperatur T2 = 2360 °K und der Leuchtdichte von 10 asb (Apostilb) ist. Bei der Farbtemperatur T1 = 2360 °K gilt also die Relation: 1 sk = 10 asb = 10/π sb.
- "Resolution 4 of the 13th CGPM (1967)". BIPM. Retrieved 2022-02-21.
- "Resolution 4: Definition of the SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. Archived from the original on 2007-06-15. Retrieved 2008-02-06.
- "Resolution 10 of the 23rd CGPM (2007)". BIPM. Retrieved 2022-02-21.
- "Unit of thermodynamic temperature (kelvin)". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 1967. Section 2.1.1.5. Archived from the original on 2007-09-26. Retrieved 2008-02-06.
- ^ Ian Mills (2010-09-29). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). BIPM. CCU. Archived from the original (PDF) on 2011-01-10. Retrieved 2011-01-01.
- "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. 2011-10-23. Archived from the original (PDF) on 2012-02-09. Retrieved 2011-10-25.
-
Wood, B. (3–4 November 2014). "Report on the Meeting of the CODATA Task Group on Fundamental Constants" (PDF). BIPM. p. 7. Archived from the original (PDF) on 2015-10-13.
Milton responded to a question about what would happen if ... the CIPM or the CGPM voted not to move forward with the redefinition of the SI. He responded that he felt that by that time the decision to move forward should be seen as a foregone conclusion.
- "2022 CODATA Value: Boltzmann constant". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "Resolution 1 of the 26th CGPM (2018)". BIPM. Retrieved 2022-02-21.
- ^ Newell, D B; Cabiati, F; Fischer, J; Fujii, K; Karshenboim, S G; Margolis, H S; de Mirandés, E; Mohr, P J; Nez, F; Pachucki, K; Quinn, T J; Taylor, B N; Wang, M; Wood, B M; Zhang, Z; et al. (Committee on Data for Science and Technology (CODATA) Task Group on Fundamental Constants) (2018-01-29). "The CODATA 2017 values of h, e, k, and NA for the revision of the SI". Metrologia. 55 (1): L13 – L16. Bibcode:2018Metro..55L..13N. doi:10.1088/1681-7575/aa950a.
- "Updating the definition of the kelvin" (PDF). BIPM. Archived from the original (PDF) on 2008-11-23. Retrieved 2010-02-23.
- Fischer, J; Fellmuth, B; Gaiser, C; Zandt, T; Pitre, L; Sparasci, F; Plimmer, M D; de Podesta, M; Underwood, R; Sutton, G; Machin, G; Gavioso, R M; Madonna Ripa, D; Steur, P P M; Qu, J; Feng, X J; Zhang, J; Moldover, M R; Benz, S P; White, D R; Gianfrani, L; Castrillo, A; Moretti, L; Darquié, B; Moufarej, E; Daussy, C; Briaudeau, S; Kozlova, O; Risegari, L; Segovia, J J; Martín, M C; del Campo, D (2018-04-01). "The Boltzmann project". Metrologia. 55 (2): R1 – R20. Bibcode:2018Metro..55R...1F. doi:10.1088/1681-7575/aaa790. PMC 6508687. PMID 31080297.
- "NIST Guide to the SI | Chapter 9: Rules and Style Conventions for Spelling Unit Names", NIST SP 811, 2016-01-28,
A derived unit is usually singular in English, for example, the value 3 m·K/W is usually spelled out as 'three square meter kelvin per watt', and the value 3 C·m/V is usually spelled out as 'three coulomb meter squared per volt'. However, a 'single' unit may be plural; for example, the value 5 kPa is spelled out as 'five kilopascals', although 'five kilopascal' is acceptable. If in such a single-unit case the number is less than one, the unit is always singular when spelled out; for example, 0.5 kPa is spelled out as 'five-tenths kilopascal'.
- "Definition of KELVIN". www.merriam-webster.com. Retrieved 2023-08-21.
- CERN English Language Style Guide (PDF). CERN. 2022. p. 64.
- "Writing with SI (Metric System) Units". NIST. 2010-01-13.
- Brady, James E.; Senese, Fred (2008-01-28). Chemistry, Student Study Guide: The Study of Matter and Its Changes. John Wiley & Sons. p. 15. ISBN 978-0-470-18464-6.
- "22.2". The Unicode Standard, Version 8.0 (PDF). Mountain View, CA, USA: The Unicode Consortium. August 2015. ISBN 978-1-936213-10-8. Archived (PDF) from the original on 2016-12-06. Retrieved 2015-09-06.
Bibliography
- Bureau International des Poids et Mesures (2019). "The International System of Units (SI) Brochure" (PDF). 9th Edition. International Committee for Weights and Measures. Retrieved 2022-04-28.
- Thomson, William (Lord Kelvin) (1882). Mathematical and physical papers: Volume I. Cambridge University Press.
External links
- Thomson, William (October 1848). "On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations". zapatopi.net. Philosophical Magazine. Archived from the original on 2008-02-01. Retrieved 2022-02-21.
- Thomson, William (March 1851). "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule's equivalent of a Thermal Unit, and M. Regnault's Observations on Steam". zapatopi.net. Transactions of the Royal Society of Edinburgh. Retrieved 2024-05-05.
| Scales of temperature | |
|---|---|
| Conversion formulas and comparison |
| SI units | |
|---|---|
| Base units | |
| Derived units with special names | |
| Other accepted units | |
| See also | |
| CGS units | |
|---|---|
| Base units | |
| Derived non EM units | |
| Derived EMU units | |
| Derived ESU units | |
 , where
, where  is the temperature in Celsius,
is the temperature in Celsius,  is the coefficient of thermal expansion, and
is the coefficient of thermal expansion, and  was "Carnot's function", a substance-independent quantity depending on temperature, motivated by an obsolete version of
was "Carnot's function", a substance-independent quantity depending on temperature, motivated by an obsolete version of  of temperature
of temperature  such that
such that  is proportional to
is proportional to  .
.
 where
where  is "the mechanical equivalent of a unit of heat", now referred to as the
is "the mechanical equivalent of a unit of heat", now referred to as the  The parameters of the scale were arbitrarily chosen to coincide with the Celsius scale at 0° and 100 °C or 273 and 373 K (the melting and boiling points of water). On this scale, an increase of approximately 222 degrees corresponds to a doubling of Kelvin temperature, regardless of the starting temperature, and "infinite cold" (
The parameters of the scale were arbitrarily chosen to coincide with the Celsius scale at 0° and 100 °C or 273 and 373 K (the melting and boiling points of water). On this scale, an increase of approximately 222 degrees corresponds to a doubling of Kelvin temperature, regardless of the starting temperature, and "infinite cold" ( is the amount of heat energy transferred into the system,
is the amount of heat energy transferred into the system,  is the heat leaving the system,
is the heat leaving the system,  is the work done by the system (
is the work done by the system ( ),
),  is the temperature of the hot reservoir in Celsius, and
is the temperature of the hot reservoir in Celsius, and  is the temperature of the cold reservoir in Celsius. The Carnot function is defined as
is the temperature of the cold reservoir in Celsius. The Carnot function is defined as  , and the absolute temperature as
, and the absolute temperature as  . One finds the relationship
. One finds the relationship  . By supposing
. By supposing  , one obtains the general principle of an absolute thermodynamic temperature scale for the Carnot engine,
, one obtains the general principle of an absolute thermodynamic temperature scale for the Carnot engine,  . The definition can be shown to correspond to the thermometric temperature of the
. The definition can be shown to correspond to the thermometric temperature of the