| Revision as of 15:38, 25 March 2020 editTop5percent (talk | contribs)268 edits →Biochemistry: separated ‘endproduct’ into ‘end’ and ‘product’Tags: Mobile edit Mobile web edit← Previous edit | Latest revision as of 05:36, 8 October 2024 edit undoPermacultura (talk | contribs)Extended confirmed users2,546 editsm →top: missing indefinite articleTags: Mobile edit Mobile app edit Android app edit App section source | ||
| (31 intermediate revisions by 23 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Attachment of a sugar to a protein or lipid}} | |||
| ⚫ | '''Glycation''' ( |
||
| {{dist|Glycosylation}} | |||
| ⚫ | '''Glycation''' ('''non-enzymatic glycosylation''') is the ] attachment of a sugar to a ], ] or ] molecule.<ref name=":0">{{Citation|last1=Lima|first1=M.|title=Glycation|date=2013-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780123786302001201|encyclopedia=Encyclopedia of Biological Chemistry (Second Edition)|pages=405–411|editor-last=Lennarz|editor-first=William J.|place=Waltham|publisher=Academic Press|language=en|doi=10.1016/b978-0-12-378630-2.00120-1|isbn=978-0-12-378631-9|access-date=2020-12-16|last2=Baynes|first2=J. W.|editor2-last=Lane|editor2-first=M. Daniel}}</ref> Typical sugars that participate in glycation are ], ], and their derivatives. Glycation is the non-enzymatic process responsible for many (e.g. micro and macrovascular) complications in ] and is implicated in some diseases and in aging.<ref>{{Cite journal| last1 = Glenn | first1 = J.| last2 = Stitt | first2 = A.| title = The role of advanced glycation end products in retinal ageing and disease| journal = Biochimica et Biophysica Acta (BBA) - General Subjects| volume = 1790| issue = 10| pages = 1109–1116| year = 2009| pmid = 19409449| doi = 10.1016/j.bbagen.2009.04.016}}</ref><ref>{{Cite journal| doi = 10.1007/BF03325227| pmid = 19448391| year = 2009| last1 = Semba | first1 = R. D.| last2 = Ferrucci| last3 = Sun| last4 = Beck| last5 = Dalal| last6 = Varadhan| last7 = Walston| last8 = Guralnik| last9 = Fried| title = Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women| volume = 21| issue = 2| pages = 182–190| journal = Aging Clinical and Experimental Research | first2 = L. | first3 = K. | first4 = J. | first5 = M. | first6 = R. | first7 = J. | first8 = J. M. | first9 = L. P.| pmc = 2684987}}</ref><ref>{{Cite journal| last1 = Semba | first1 = R.| last2 = Najjar | first2 = S.| last3 = Sun | first3 = K.| last4 = Lakatta | first4 = E.| last5 = Ferrucci | first5 = L.| title = Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults| journal = American Journal of Hypertension| volume = 22| issue = 1| pages = 74–79| year = 2009| pmid = 19023277| doi = 10.1038/ajh.2008.320| pmc = 2637811}}</ref> Glycation end products are believed to play a causative role in the vascular complications of ].<ref>{{Cite journal| pmid = 18331228| year = 2007| last1 = Yan | first1 = S. F.| last2 = D'Agati| last3 = Schmidt| last4 = Ramasamy| title = Receptor for Advanced Glycation Endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging| volume = 7| issue = 8| pages = 699–710| journal = Current Molecular Medicine| doi = 10.2174/156652407783220732 | first2 = V. | first3 = A. M. | first4 = R.}}</ref> | ||
| ⚫ | In contrast with glycation, ] is the enzyme-mediated ATP-dependent attachment of sugars to protein or lipid. Glycosylation occurs at defined sites on the target molecule. It is a common form of ] of proteins and is required for the functioning of the mature protein. | ||
| ⚫ | In contrast with glycation, ] is the enzyme-mediated ATP-dependent attachment of sugars to a protein or lipid.<ref name=":0" /> Glycosylation occurs at defined sites on the target molecule. It is a common form of ] of proteins and is required for the functioning of the mature protein. | ||
| ==Biochemistry== | ==Biochemistry== | ||
| ] | ] (in HbA1c, R is typically N-terminal valine)<ref>{{cite journal|title=Chemistry of Amadori Rearrangement Products: Analysis, Synthesis, Kinetics, Reactions, and Spectroscopic Properties|author1= Yaylayan, Varoujan A. |author2=Huyghues-Despointes, Alexis |journal=Critical Reviews in Food Science and Nutrition|year=1994|volume=34|issue= 4|pages=321–69|doi=10.1080/10408399409527667|pmid= 7945894}}</ref>]] | ||
| ]s (R = CH<sub>2</sub>CH(OH)CH(OH)CH<sub>2</sub>OH) are typical glycation products. They arise by the condensation of 3-deoxyglucosone with the guanidine group of an ] residue.<ref name=DRCP>{{cite journal|journal=Diabetes Research and Clinical Practice|volume=148|year=2019 |
]s (R = CH<sub>2</sub>CH(OH)CH(OH)CH<sub>2</sub>OH) are typical glycation products. They arise by the condensation of 3-deoxyglucosone with the guanidine group of an ] residue.<ref name=DRCP>{{cite journal|journal=Diabetes Research and Clinical Practice|volume=148|year=2019|title=Methylglyoxal, a Potent Inducer of AGEs, Connects between Diabetes and Cancer|first1=Justine|last1=Bellier|first2=Marie-Julie|last2=Nokin|first3=Eva|last3= Lardé|first4=Philippe|last4=Karoyan|first5=Olivier|last5=Peulen|first6=Vincent|last6=Castronovo|first7=Akeila|last7=Bellahcène|pages=200–211|doi=10.1016/j.diabres.2019.01.002|pmid=30664892|s2cid=58631777 }}</ref>]] | ||
| Glycations occur mainly in the bloodstream to a small proportion of the absorbed simple sugars: ], ], and ]. It appears that fructose has approximately ten times the glycation activity of glucose, the primary body fuel.<ref>{{cite journal |doi=10.1021/bi00406a016 |vauthors=McPherson JD, Shilton BH, Walton DJ |title=Role of fructose in glycation and cross-linking of proteins |journal=Biochemistry |volume=27 |issue=6 |pages=1901–7 |date=March 1988 |pmid=3132203}}</ref> Glycation can occur through ]s, ]s, and ]s; which lead to ]s (AGEs). | Glycations occur mainly in the bloodstream to a small proportion of the absorbed simple sugars: ], ], and ]. It appears that fructose has approximately ten times the glycation activity of glucose, the primary body fuel.<ref>{{cite journal |doi=10.1021/bi00406a016 |vauthors=McPherson JD, Shilton BH, Walton DJ |title=Role of fructose in glycation and cross-linking of proteins |journal=Biochemistry |volume=27 |issue=6 |pages=1901–7 |date=March 1988 |pmid=3132203}}</ref> Glycation can occur through ]s, ]s, and ]s; which lead to ]s (AGEs).<ref name=":0" /> | ||
| ==Biomedical implications== | ==Biomedical implications== | ||
| Red blood cells have a consistent lifespan of 120 days and are accessible for measurement of ]. Measurement of ]—the predominant form of glycated hemoglobin—enables medium-term blood sugar control to be monitored in ]. | Red blood cells have a consistent lifespan of 120 days and are accessible for measurement of ]. Measurement of ]—the predominant form of glycated hemoglobin—enables medium-term blood sugar control to be monitored in ]. | ||
| Some glycation |
Some glycation products are implicated in many age-related chronic diseases, including ]s (the endothelium, fibrinogen, and collagen are damaged) and ] (amyloid proteins are side-products of the reactions progressing to AGEs).<ref>{{cite journal|last=Münch|first=Gerald|title=Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of β-amyloid peptide|journal=Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease|date=27 February 1997|volume=1360|issue=1|pages=17–29|doi=10.1016/S0925-4439(96)00062-2|pmid=9061036|display-authors=etal|doi-access=}}</ref><ref>{{cite journal|last=Munch|first=G|author2=Deuther-Conrad W |author3=Gasic-Milenkovic J. |title=Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer's disease--a target for neuroprotective treatment strategies?|journal=J Neural Transm Suppl|year=2002|volume=62|pages=303–307|pmid= 12456073|issue=62|doi=10.1007/978-3-7091-6139-5_28}}</ref> | ||
| ⚫ | Long-lived cells (such as nerves and different types of brain cell), long-lasting proteins (such as ]s of the ] and ]), and DNA can sustain substantial glycation over time. Damage by glycation results in stiffening of the collagen in the blood vessel walls, leading to high blood pressure, especially in diabetes.<ref>{{cite journal|last=Soldatos|first=G.|author2=Cooper ME|title=Advanced glycation end products and vascular structure and function|journal=Curr Hypertens Rep|date=Dec 2006|volume=8|issue=6|pages=472–478|pmid=17087858|doi=10.1007/s11906-006-0025-8|s2cid=31239347}}</ref> Glycations also cause weakening of the collagen in the blood vessel walls,<ref>{{cite journal|last=Lee|first=J. Michael|author2=Samuel P. Veres|title=Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon|journal=Journal of Applied Physiology|date=2019-04-02|volume=126|issue=4|pages=832–841|doi=10.1152/japplphysiol.00430.2018|pmid=30653412|pmc=6485690}}</ref> which may lead to micro- or macro-aneurysm; this may cause strokes if in the brain. | ||
| ==DNA glycation== | |||
| ⚫ | Long-lived cells (such as nerves and different types of brain cell), long-lasting proteins (such as ]s of the ] and ]), and DNA can sustain substantial glycation over time. Damage by glycation results in stiffening of the collagen in the blood vessel walls, leading to high blood pressure, especially in diabetes.<ref>{{cite journal|last=Soldatos|first=G.|author2=Cooper ME|title=Advanced glycation end products and vascular structure and function|journal=Curr Hypertens Rep|date=Dec 2006|volume=8|issue=6|pages=472–478|pmid=17087858|doi=10.1007/s11906-006-0025-8}}</ref> Glycations also cause weakening of the collagen in the blood vessel walls,{{ |
||
| The term DNA glycation applies to ] induced by reactive carbonyls (principally ] and ]) that are present in cells as by-products of sugar metabolism.<ref name = Richarme2017>Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart JC, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 2017 Jul 14;357(6347):208-211. doi: 10.1126/science.aag1095. Epub 2017 Jun 8. PMID 28596309</ref> Glycation of DNA can cause ], breaks in DNA and ].<ref name = Richarme2017/> ] in DNA is the base most susceptible to glycation. Glycated DNA, as a form of damage, appears to be as frequent as the more well studied oxidative DNA damage. A protein, designated DJ-1 (also known as ]), is employed in the repair of glycated DNA bases in humans, and homologs of this protein have also been identified in bacteria.<ref name = Richarme2017/> | |||
| ==See also== | ==See also== | ||
| *] | * ] | ||
| *] | * ] | ||
| *] | * ] | ||
| *] | * ] | ||
| *] | * ] | ||
| *] | * ] | ||
| * ] | |||
| *] | * ] | ||
| ==Additional reading== | ==Additional reading== | ||
| *{{cite journal |vauthors=Ahmed N, Furth AJ |title=Failure of common glycation assays to detect glycation by fructose |journal=Clin. Chem. |volume=38 |issue=7 |pages=1301–3 |date=July 1992 |pmid=1623595}} | * {{cite journal |vauthors=Ahmed N, Furth AJ |title=Failure of common glycation assays to detect glycation by fructose |journal=Clin. Chem. |volume=38 |issue=7 |pages=1301–3 |date=July 1992 |doi=10.1093/clinchem/38.7.1301 |pmid=1623595|doi-access=free }} | ||
| *{{cite journal |author=Vlassara H |title=Advanced glycation in health and disease: role of the modern environment |journal=Annals of the New York Academy of Sciences |volume=1043 |issue= 1|pages=452–60 |date=June 2005 |pmid=16037266 |doi=10.1196/annals.1333.051|bibcode = 2005NYASA1043..452V }} | * {{cite journal |author=Vlassara H |title=Advanced glycation in health and disease: role of the modern environment |journal=Annals of the New York Academy of Sciences |volume=1043 |issue= 1|pages=452–60 |date=June 2005 |pmid=16037266 |doi=10.1196/annals.1333.051|bibcode = 2005NYASA1043..452V |s2cid=20952378 }} | ||
| ==References== | ==References== | ||
| {{reflist|2}} | {{reflist|2}} | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 05:36, 8 October 2024
Attachment of a sugar to a protein or lipid Not to be confused with Glycosylation.Glycation (non-enzymatic glycosylation) is the covalent attachment of a sugar to a protein, lipid or nucleic acid molecule. Typical sugars that participate in glycation are glucose, fructose, and their derivatives. Glycation is the non-enzymatic process responsible for many (e.g. micro and macrovascular) complications in diabetes mellitus and is implicated in some diseases and in aging. Glycation end products are believed to play a causative role in the vascular complications of diabetes mellitus.
In contrast with glycation, glycosylation is the enzyme-mediated ATP-dependent attachment of sugars to a protein or lipid. Glycosylation occurs at defined sites on the target molecule. It is a common form of post-translational modification of proteins and is required for the functioning of the mature protein.
Biochemistry
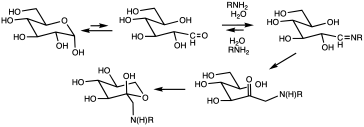

Glycations occur mainly in the bloodstream to a small proportion of the absorbed simple sugars: glucose, fructose, and galactose. It appears that fructose has approximately ten times the glycation activity of glucose, the primary body fuel. Glycation can occur through Amadori reactions, Schiff base reactions, and Maillard reactions; which lead to advanced glycation end products (AGEs).
Biomedical implications
Red blood cells have a consistent lifespan of 120 days and are accessible for measurement of glycated hemoglobin. Measurement of HbA1c—the predominant form of glycated hemoglobin—enables medium-term blood sugar control to be monitored in diabetes.
Some glycation products are implicated in many age-related chronic diseases, including cardiovascular diseases (the endothelium, fibrinogen, and collagen are damaged) and Alzheimer's disease (amyloid proteins are side-products of the reactions progressing to AGEs).
Long-lived cells (such as nerves and different types of brain cell), long-lasting proteins (such as crystallins of the lens and cornea), and DNA can sustain substantial glycation over time. Damage by glycation results in stiffening of the collagen in the blood vessel walls, leading to high blood pressure, especially in diabetes. Glycations also cause weakening of the collagen in the blood vessel walls, which may lead to micro- or macro-aneurysm; this may cause strokes if in the brain.
DNA glycation
The term DNA glycation applies to DNA damage induced by reactive carbonyls (principally methylglyoxal and glyoxal) that are present in cells as by-products of sugar metabolism. Glycation of DNA can cause mutation, breaks in DNA and cytotoxicity. Guanine in DNA is the base most susceptible to glycation. Glycated DNA, as a form of damage, appears to be as frequent as the more well studied oxidative DNA damage. A protein, designated DJ-1 (also known as PARK7), is employed in the repair of glycated DNA bases in humans, and homologs of this protein have also been identified in bacteria.
See also
- Advanced glycation end-product
- Alagebrium
- Fructose
- Galactose
- Glucose
- Glycosylation
- Glycated hemoglobin
- List of aging processes
Additional reading
- Ahmed N, Furth AJ (July 1992). "Failure of common glycation assays to detect glycation by fructose". Clin. Chem. 38 (7): 1301–3. doi:10.1093/clinchem/38.7.1301. PMID 1623595.
- Vlassara H (June 2005). "Advanced glycation in health and disease: role of the modern environment". Annals of the New York Academy of Sciences. 1043 (1): 452–60. Bibcode:2005NYASA1043..452V. doi:10.1196/annals.1333.051. PMID 16037266. S2CID 20952378.
References
- ^ Lima, M.; Baynes, J. W. (2013-01-01), "Glycation", in Lennarz, William J.; Lane, M. Daniel (eds.), Encyclopedia of Biological Chemistry (Second Edition), Waltham: Academic Press, pp. 405–411, doi:10.1016/b978-0-12-378630-2.00120-1, ISBN 978-0-12-378631-9, retrieved 2020-12-16
- Glenn, J.; Stitt, A. (2009). "The role of advanced glycation end products in retinal ageing and disease". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1109–1116. doi:10.1016/j.bbagen.2009.04.016. PMID 19409449.
- Semba, R. D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J. M.; Fried, L. P. (2009). "Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women". Aging Clinical and Experimental Research. 21 (2): 182–190. doi:10.1007/BF03325227. PMC 2684987. PMID 19448391.
- Semba, R.; Najjar, S.; Sun, K.; Lakatta, E.; Ferrucci, L. (2009). "Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults". American Journal of Hypertension. 22 (1): 74–79. doi:10.1038/ajh.2008.320. PMC 2637811. PMID 19023277.
- Yan, S. F.; D'Agati, V.; Schmidt, A. M.; Ramasamy, R. (2007). "Receptor for Advanced Glycation Endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging". Current Molecular Medicine. 7 (8): 699–710. doi:10.2174/156652407783220732. PMID 18331228.
- Yaylayan, Varoujan A.; Huyghues-Despointes, Alexis (1994). "Chemistry of Amadori Rearrangement Products: Analysis, Synthesis, Kinetics, Reactions, and Spectroscopic Properties". Critical Reviews in Food Science and Nutrition. 34 (4): 321–69. doi:10.1080/10408399409527667. PMID 7945894.
- Bellier, Justine; Nokin, Marie-Julie; Lardé, Eva; Karoyan, Philippe; Peulen, Olivier; Castronovo, Vincent; Bellahcène, Akeila (2019). "Methylglyoxal, a Potent Inducer of AGEs, Connects between Diabetes and Cancer". Diabetes Research and Clinical Practice. 148: 200–211. doi:10.1016/j.diabres.2019.01.002. PMID 30664892. S2CID 58631777.
- McPherson JD, Shilton BH, Walton DJ (March 1988). "Role of fructose in glycation and cross-linking of proteins". Biochemistry. 27 (6): 1901–7. doi:10.1021/bi00406a016. PMID 3132203.
- Münch, Gerald; et al. (27 February 1997). "Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of β-amyloid peptide". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1360 (1): 17–29. doi:10.1016/S0925-4439(96)00062-2. PMID 9061036.
- Munch, G; Deuther-Conrad W; Gasic-Milenkovic J. (2002). "Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer's disease--a target for neuroprotective treatment strategies?". J Neural Transm Suppl. 62 (62): 303–307. doi:10.1007/978-3-7091-6139-5_28. PMID 12456073.
- Soldatos, G.; Cooper ME (Dec 2006). "Advanced glycation end products and vascular structure and function". Curr Hypertens Rep. 8 (6): 472–478. doi:10.1007/s11906-006-0025-8. PMID 17087858. S2CID 31239347.
- Lee, J. Michael; Samuel P. Veres (2019-04-02). "Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon". Journal of Applied Physiology. 126 (4): 832–841. doi:10.1152/japplphysiol.00430.2018. PMC 6485690. PMID 30653412.
- ^ Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart JC, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 2017 Jul 14;357(6347):208-211. doi: 10.1126/science.aag1095. Epub 2017 Jun 8. PMID 28596309