| Revision as of 21:06, 16 December 2006 editDrMicro (talk | contribs)19,885 edits →Infections in non primate mammals← Previous edit | Latest revision as of 06:32, 20 August 2024 edit undoDocWatson42 (talk | contribs)Extended confirmed users, Pending changes reviewers217,897 edits Cleaned up image placement (to prevent the overrunning of the appendices in wide browser windows) and other matters, including references and deleting cruft. | ||
| Line 1: | Line 1: | ||
| {{Short description|Genus of parasitic protists that can cause malaria}} | |||
| ]]] | |||
| {{For|the multinucleate stage of some microorganisms|Plasmodium (life cycle)}} | |||
| :''A '''plasmodium'''] is also the macroscopic form of the unusual ] known as ]s.'' | |||
| {{Good article}} | |||
| {{Taxobox | color = khaki | |||
| {{Automatic taxobox | |||
| | name = ''Plasmodium'' | |||
| | image = Malaria.jpg |
| image = Malaria.jpg | ||
| | image_alt = False-colored electron micrograph of a sporozoite | |||
| | regnum = ]a | |||
| | image_caption = False-colored ] of a ] | |||
| | phylum = ] | |||
| | taxon = Plasmodium | |||
| | classis = ] | |||
| | authority = ] & ], 1885 | |||
| | ordo = ] | |||
| | synonyms_ref = <!-- | |||
| | familia = ] | |||
| | subdivision_ranks = Subgenera and species<ref>{{cite web|title=''Plasmodium''|url=https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Undef&id=5820&lvl=3&srchmode=1&keep=1&unlock|website=NCBI taxonomy|publisher=National Center for Biotechnology Information|access-date=5 January 2019|location=Bethesda, MD|language=en|format=HTML}}</ref> | |||
| | genus = '''''Plasmodium''''' | |||
| | subdivision_ranks = Species | |||
| | subdivision = | | subdivision = | ||
| '']'' |
* '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' | ** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
* '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| *Species '']'' | |||
| '']''<br/> | |||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' | ||
| ''] |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| '']'' |
** '']'' | ||
| ''] |
** '']'' --> | ||
| '']''<br/> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']</br> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br> | |||
| '']''</br> | |||
| '']''<br/> | |||
| '']''</br> | |||
| '']''<br> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| '']''<br> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''</br> | |||
| '']''<br/> | |||
| '']''</br> | |||
| '']''<br/> | |||
| '']''<br/> | |||
| etc. | |||
| }} | }} | ||
| '''''Plasmodium''''' is a ] of unicellular ]s that are ]s of ]s and ]s. The life cycles of ''Plasmodium'' species involve development in a ] insect ] which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue (often the liver) before entering the bloodstream to infect ]s. The ensuing destruction of host red blood cells can result in ]. During this infection, some parasites are picked up by a blood-feeding insect (]es in majority cases), continuing the life cycle.<ref name=CDCAbout>{{cite web |title=CDC – Malaria Parasites – About |url=https://www.cdc.gov/malaria/about/biology/parasites.html |website=CDC: Malaria |publisher=U.S. Centers for Disease Control and Prevention |access-date=28 December 2015}}</ref> | |||
| '''''Plasmodium''''' is a genus of parasitic ]. Infection with this genus is known as ]. The parasite always has two hosts in its ]: a ] ] and a ] host. At least ten species infect humans. Other species infect other animals, including ]s, ]s and ]s. | |||
| ''Plasmodium'' is a member of the phylum ], a large group of parasitic eukaryotes. Within Apicomplexa, ''Plasmodium'' is in the order ] and family ]. Over 200 species of ''Plasmodium'' have been described, many of which have been subdivided into 14 subgenera based on parasite morphology and host range. Evolutionary relationships among different ''Plasmodium'' species do not always follow taxonomic boundaries; some species that are morphologically similar or infect the same host turn out to be distantly related. | |||
| ==Taxonomy and host range == | |||
| Species of ''Plasmodium'' are distributed globally wherever suitable hosts are found. Insect hosts are most frequently ]es of the genera '']'' and '']''. Vertebrate hosts include reptiles, birds, and mammals. ''Plasmodium'' parasites were first identified in the late 19th century by ]. Over the course of the 20th century, many other species were discovered in various hosts and classified, including five species that regularly infect humans: '']'', '']'', '']'', '']'', and '']''. ''P. falciparum'' is by far the most lethal in humans, resulting in hundreds of thousands of deaths per year. A number of ] have been developed to treat ''Plasmodium'' infection; however, the parasites have evolved resistance to each drug developed. | |||
| The genus ''Plasmodium'' was created in ] by ] and ] and there are over 175 species currently recognised in this genus. The genus is currently (]) in need of reorganisation as it has been shown that other parasites belonging to the genera '']'' and ] appear to be closely related to this genus. It is likely that other species such as '']'' will be included in this genus once it is revised. | |||
| Although the parasite can also infect people via ], this is very rare, and ''Plasmodium'' cannot be spread from person to person. Some of subspecies of ''Plasmodium'' are ]. | |||
| Host range among the mammalian orders is non uniform. At least 25 ] infect ]; ] outside the tropical parts of ] are rarely affected; a few species are known to infect ]s, ]s and ]s; while ]s, ]s and ]s are not known to act as hosts. | |||
| == Description == | |||
| == Discovery of the life cycle == | |||
| ] but with unusual features.]] | |||
| The genus ''Plasmodium'' consists of all ]s in the phylum Apicomplexa that both undergo the asexual replication process of ] inside host ]s and produce the crystalline pigment ] as a byproduct of digesting host ].<ref name=TOL/> ''Plasmodium'' species contain many features that are common to other eukaryotes, and some that are unique to their phylum or genus. The ''Plasmodium'' ] is separated into 14 ] contained in the ]. ''Plasmodium'' parasites maintain ] of their genome through much of the life cycle, ] the genome only for a brief sexual exchange within the ] of the insect host.<ref name=Obado>{{cite journal|doi=10.1016/j.molbiopara.2016.07.008|pmid=27475118|title=The nuclear envelope and gene organization in parasitic protozoa: Specializations associated with disease|journal=Molecular and Biochemical Parasitology|volume=209|issue=1–2|pages=104–113|year=2016|last1=Obado|first1=Samson O|last2=Glover|first2=Lucy |last3=Deitsch |first3=Kirk W.}}</ref> Attached to the nucleus is the ] (ER), which functions similarly to the ER in other eukaryotes. Proteins are trafficked from the ER to the ] which generally consists of a single membrane-bound compartment in Apicomplexans.<ref name=Ruiz>{{cite journal|doi= 10.1016/j.molbiopara.2016.01.007|pmid= 26844642|pmc= 5154328|title= Vacuolar protein sorting mechanisms in apicomplexan parasites|journal= Molecular and Biochemical Parasitology|volume= 209|issue= 1–2|pages= 18–25|year= 2016|last1= Jimenez-Ruiz|first1= Elena|last2= Morlon-Guyot|first2= Juliette|last3= Daher|first3= Wassim|last4= Meissner|first4= Markus}}</ref> From here, proteins are trafficked to various cellular compartments or to the cell surface.<ref name=Ruiz/> | |||
| In ] ] demonstrated the existence of ''Plasmodium'' in the wall of the ] and ] of a '']'' ]. For this discovery he won the ] in ]. However credit must also be given to the Italian professor ], who showed that human malaria could only be transmitted by '']'' mosquitoes. | |||
| Like other apicomplexans, ''Plasmodium'' species have several cellular structures at the ] end of the parasite that serve as specialized organelles for secreting effectors into the host. The most prominent are the bulbous ] which contain parasite proteins involved in invading the host cell and modifying the host once inside.<ref>{{cite journal|doi=10.1016/j.pt.2013.03.003 |pmid=23570755 |title=Plasmodium rhoptry proteins: Why order is important|journal=Trends in Parasitology |volume=29 |issue=5|pages=228–36|year=2013|last1=Counihan|first1=Natalie A. |last2=Kalanon |first2=Ming |last3=Coppel |first3=Ross L.|last4=De Koning-Ward|first4=Tania F.}}</ref> Adjacent to the rhoptries are smaller structures termed ]s that contain parasite proteins required for motility as well as recognizing and attaching to host cells.<ref name=Kemp2013>{{cite journal|doi=10.1111/1574-6976.12013|pmid=23186105|title=Subversion of host cellular functions by the apicomplexan parasites|journal=FEMS Microbiology Reviews|volume=37|issue=4|pages=607–31|year=2013|last1=Kemp|first1=Louise E.|last2=Yamamoto|first2=Masahiro|last3=Soldati-Favre|first3=Dominique|url=https://archive-ouverte.unige.ch/unige:28792/ATTACHMENT01|doi-access=free}}</ref> Spread throughout the parasite are secretory ]s called ] that contain parasite proteins involved in modifying the membrane that separates the parasite from the host, termed the ].<ref name=Kemp2013/> | |||
| The species of ''Plasmodium'' that parasitise humans include: | |||
| Species of ''Plasmodium'' also contain two large membrane-bound organelles of ], the ] and the ], both of which play key roles in the parasite's ]. Unlike mammalian cells which contain many mitochondria, ''Plasmodium'' cells contain a single large mitochondrion that coordinates its division with that of the ''Plasmodium'' cell.<ref name=Sheiner2013>{{cite journal |doi=10.1016/j.mib.2013.07.003|pmid=23927894|title=The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp|journal=Current Opinion in Microbiology|volume=16|issue=4 |pages=452–8|year=2013|last1=Sheiner |first1=Lilach |last2=Vaidya |first2=Akhil B.|last3=McFadden|first3=Geoffrey I.|pmc=3767399}}</ref> Like in other eukaryotes, the ''Plasmodium'' mitochondrion is capable of generating energy in the form of ] via the ]; however, this function is only required for parasite survival in the insect host, and is not needed for growth in red blood cells.<ref name=Sheiner2013/> A second organelle, the apicoplast, is derived from a ] event, in this case the acquisition of a ] by the ''Plasmodium'' ancestor.<ref name=McFadden2016>{{cite journal|doi=10.1016/j.ijpara.2016.08.005|pmid=27773518|title=The apicoplast: Now you see it, now you don't|journal=International Journal for Parasitology|volume=47|issue=2–3|pages=137–144 |year=2017|last1=McFadden|first1=Geoffrey Ian|last2=Yeh|first2=Ellen|pmc=5406208}}</ref> The apicoplast is involved in the synthesis of various metabolic precursors, including ]s, ]s, ]s, and components of the ] biosynthesis pathway.<ref>{{cite journal|last1=Dooren|first1=Giel|last2=Striepen |first2=Boris |title=The Algal Past and Parasite Present of the Apicoplast|journal=Annual Review of Microbiology|date=June 26, 2013|volume=67|pages=271–289|doi=10.1146/annurev-micro-092412-155741|pmid=23808340}}</ref> | |||
| *'']'' (the cause of malignant tertian malaria) | |||
| *'']'' (the most frequent cause of benign tertian malaria) | |||
| *'']'' (the other, less frequent, cause of benign tertian malaria) | |||
| *'']'' (the cause of benign quartan malaria) | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| *'']'' | |||
| ==Life cycle== | |||
| The first four listed here are the most common species that infect humans. With the use of the ] additional species have been and are still being identified that infect humans. | |||
| ] | |||
| ])]] | |||
| The life cycle of ''Plasmodium'' involves several distinct stages in the insect and vertebrate ]. Parasites are generally introduced into a vertebrate host by the bite of an insect host (generally a mosquito, with the exception of some ''Plasmodium'' species of reptiles).<ref name=Sullivan>{{cite book|title=Malaria: Drugs, Disease, and Post-genomic Biology |date=2005 |editor1=Sullivan, D |editor2=Krishna, S. |publisher=Springer |isbn=978-3-540-29088-9 |chapter=Molecular Genetics of Mosquito Resistance to Malaria Parasites |author1=Vernick, K.D. |author2=Oduol, F. |author3=Lazarro, B.P. |author4=Glazebrook, J. |author5=Xu, J. |author6=Riehle, M. |author7=Li, J. |page=384}}</ref> Parasites first infect the liver or other tissue, where they undergo a single large round of replication before exiting the host cell to infect ].<ref name=CDCBiology>{{cite web |title=CDC – Malaria Parasites – Biology|url=https://www.cdc.gov/malaria/about/biology/index.html |website=CDC: Malaria |publisher=U.S. Centers for Disease Control and Prevention |access-date=28 December 2015}}</ref> At this point, some species of ''Plasmodium'' of primates can form a long-lived dormant stage called a hypnozoite,<ref>{{cite journal | author=Markus, M. B. | title = Malaria: Origin of the Term 'Hypnozoite' | journal = Journal of the History of Biology | volume = 44 | issue = 4 | pages = 781–786 | date = 2011 | pmid = 20665090 | doi = 10.1007/s10739-010-9239-3| s2cid = 1727294 }}</ref> which can remain in the liver for more than a year.<ref>{{cite journal|doi=10.1101/cshperspect.a025486 |pmid=28242785 |title=Malaria Parasite Liver Infection and Exoerythrocytic Biology |journal=Cold Spring Harbor Perspectives in Medicine |volume=7 |issue=6 |pages=a025486 |year=2017 |last1=Vaughan |first1=Ashley M. |last2=Kappe |first2=Stefan H. I. |pmc=5453383 }}</ref> However, for most ''Plasmodium'' species, the parasites in infected liver cells are only what are called merozoites. After emerging from the liver, they enter red blood cells, as explained above. They then go through continuous cycles of erythrocyte infection, while a small percentage of parasites differentiate into a sexual stage called a gametocyte which is picked up by an insect host taking a blood meal. In some hosts, invasion of erythrocytes by ''Plasmodium'' species can result in disease, called malaria. This can sometimes be severe, rapidly followed by death of the host (e.g. ''P. falciparum'' in humans). In other hosts, ''Plasmodium'' infection can apparently be asymptomatic.<ref name=Sullivan/> | |||
| == Taxonomy == | |||
| Even when humans have such subclinical plasmodial infections, there can nevertheless be very large numbers of multiplying parasites concealed in, particularly, the spleen and bone marrow. Certainly, this applies in the case of ''P. vivax''. These hidden parasites (in addition to hypnozoites) are thought to be the origin of instances of recurrent ''P. vivax'' malaria.<ref>{{cite journal |last1=Markus |first1=M. B. |title=Theoretical origin of genetically homologous Plasmodium vivax malarial recurrences |journal=Southern African Journal of Infectious Diseases |date=2022 |volume=37 |issue=1 |page=369 |doi=10.4102/sajid.v37i1.369 |pmid=35399558 |pmc=8991251}}</ref> | |||
| ''Plasmodium'' belongs to the ] ] (Levine, ]), ] ] and ] ]. There are currently 450 recognised ] in this order. Many species of this order are undergoing reexamination of thier taxonomy with ] analysis. It seems likely that many of these species will be re assigned after these studies have been completed. <ref name=Perkins2002> Perkins SL, Schall JJ (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitology 88 (5): 972-978 </ref> <ref name=Yotoko2006> Yotoko, K. S. C. and Elisei C. (2006) Malaria parasites (Apicomplexa, Haematozoea) and their relationships with their hosts: is there an evolutionary cost for the specialization? J. Zoo. Syst. Evol. Res. 44 (4) 265 </ref> For this reason the entire order is outlined here. | |||
| ]s, one of several different forms of the parasite, from a mosquito]] | |||
| Within the red blood cells, the merozoites grow first to a ring-shaped form and then to a larger form called a ]. Trophozoites then mature to ]s which divide several times to produce new merozoites. The infected red blood cell eventually bursts, allowing the new merozoites to travel within the bloodstream to infect new red blood cells. Most merozoites continue this replicative cycle, however some merozoites upon infecting red blood cells differentiate into male or female sexual forms called gametocytes. These gametocytes circulate in the blood until they are taken up when a mosquito feeds on the infected vertebrate host, taking up blood which includes the gametocytes.<ref name=CDCBiology/> | |||
| *Order Haemosporida | |||
| In the mosquito, the gametocytes move along with the ] to the mosquito's midgut. Here the ]s develop into male and female ]s which ] each other, forming a ]. Zygotes then develop into a motile form called an ], which penetrates the wall of the midgut. Upon traversing the midgut wall, the ookinete embeds into the gut's exterior membrane and develops into an oocyst. Oocysts divide many times to produce large numbers of small elongated ]s. These sporozoites migrate to the salivary glands of the mosquito where they can be injected into the blood of the next host the mosquito bites, repeating the cycle.<ref name=CDCBiology/> | |||
| ==Evolution and taxonomy== | |||
| *Family '']''</br> | |||
| ]'', 15–20 million years old]] | |||
| *Genus '']''</br> | |||
| *Subgenus '']''</br> | |||
| *Subgenus '']''</br> | |||
| === Taxonomy === | |||
| ''Plasmodium'' belongs to the ] ], a taxonomic group of single-celled parasites with characteristic ] at one end of the cell.<ref name=Morrison2009>{{cite journal |doi=10.1016/j.pt.2009.05.010 |pmid=19635681 |title=Evolution of the Apicomplexa: Where are we now? |journal=Trends in Parasitology |volume=25 |issue=8 |pages=375–82 |year=2009 |last1=Morrison |first1=David A.}}</ref> Within Apicomplexa, ''Plasmodium'' is within the ] ], a group that includes all apicomplexans that live within blood cells.<ref>{{cite web|url=http://tolweb.org/Haemosporina/124976 |access-date=1 May 2018 |title=Haemospororida Danielewski 1885 |author=Votypka J |website=Tree of Life}}</ref> Based on the presence of the pigment ] and the method of ], the order is further split into four families, of which ''Plasmodium'' is in the ] ].<ref name=Perkins2014/> | |||
| The genus ''Plasmodium'' consists of over 200 species, generally described on the basis of their appearance in blood smears of infected vertebrates.<ref name=Martinsen2013>{{cite book |title=Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology |editor1=Carlton, J.M. |editor2=Perkins, S.L. |editor3=Deitsch, K.W. |isbn=978-1908230072 |publisher=Caister Academic Press |date=2013 |chapter=The Diversity of ''Plasmodium'' and other Haemosporidians: The Intersection of Taxonomy, Phylogenetics, and Genomics |author1=Martinsen, E. S. |author2=Perkins, S. L. |pages=1–15}}</ref> These species have been categorized on the basis of their morphology and host range into 14 subgenera:<ref name=Perkins2014>{{cite journal|title=Malaria's Many Mates: Past, Present, and Future of the Systematics of the Order Haemosporida |author=Perkins, S. L. |date=2014 |journal=Journal of Parasitology |volume=100 |issue=1 |pages=11–25 |doi=10.1645/13-362.1|pmid=24059436 |s2cid=21291855 }}</ref> | |||
| *Family '']''</br> | |||
| *Genus ''Plasmodium''</br> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| *Subgenus '']''<br/> | |||
| * Subgenus '']'' <small>(Telford, 1988)</small> – reptiles | |||
| * Subgenus '']'' <small>(Valkiunas, 1997)</small> – birds | |||
| * Subgenus '']'' <small>(Garnham, 1966)</small> – reptiles | |||
| * Subgenus '']'' <small>(Corradetti, et al. 1963)</small> – birds | |||
| * Subgenus '']'' <small>(Corradetti, et al. 1963)</small> – birds | |||
| * Subgenus '']'' <small>(Corradetti, et al. 1963)</small> – birds | |||
| * Subgenus '']'' <small>(Telford, 1988)</small> – reptiles | |||
| * Subgenus '']'' <small>(Bray, 1958)</small> – great apes, humans | |||
| * Subgenus '']'' <small>(Corradetti, et al. 1963)</small> – birds | |||
| * Subgenus '']'' <small>(Telford, 1988)</small> – reptiles | |||
| * Subgenus '']'' <small>(Telford, 1988)</small> – reptiles | |||
| * Subgenus ''Plasmodium'' <small>(Bray, 1955)</small> – monkeys and apes | |||
| * Subgenus '']'' <small>(Garnham, 1966)</small> – reptiles | |||
| * Subgenus '']'' <small>(Garnham, 1964)</small> – mammals inc. primates | |||
| Species infecting ]s and ] with the exceptions of ''P. falciparum'' and ''P. reichenowi'' (which together make up the subgenus ''Laverania'') are classified in the subgenus ''Plasmodium''. Parasites infecting other ]s including some primates (]s and others) are classified in the subgenus ''Vinckeia''. The five subgenera ''Bennettinia'', ''Giovannolaia'', ''Haemamoeba'', ''Huffia'', and ''Novyella'' contain the known avian malarial species.<ref name=Valkiunas4>{{cite book |title=Avian Malaria Parasites and Other Haemosporidia |author=Valkiunas, Gediminas |publisher=CRC Press |isbn=9780415300971 |date=2004 |chapter=Brief Historical Summary |pages=9–15}}</ref> The remaining subgenera: ''Asiamoeba'', ''Carinamoeba'', ''Lacertamoeba'', ''Ophidiella'', ''Paraplasmodium'', and ''Sauramoeba'' contain the diverse groups of parasites found to infect reptiles.<ref name="Telford1988"/> | |||
| *Family ''] | |||
| *Genus '']''</br> | |||
| *Subgenus '']''</br> | |||
| ===Phylogeny=== | |||
| More recent studies of ''Plasmodium'' species using molecular methods have implied that the group's evolution has not perfectly followed taxonomy.<ref name=TOL>{{cite web |url=http://tolweb.org/Plasmodium/68071 |access-date=1 June 2016 |title=''Plasmodium'' |publisher=Tree of Life Web Project |author1=Zilversmit, M. |author2=Perkins, S.}}</ref> Many ''Plasmodium'' species that are morphologically similar or infect the same hosts turn out to be only distantly related.<ref name=Rich2003>{{cite book |doi=10.1016/S0065-308X(03)54005-2 |pmid=14711087 |title=Progress in Malaria Research: the Case for Phylogenetics |volume=54 |pages=255–80 |year=2003 |last1=Rich |first1=S. |last2=Ayala |first2=F |series=Advances in Parasitology |isbn=978-0-12-031754-7}}</ref> In the 1990s, several studies sought to evaluate evolutionary relationships of ''Plasmodium'' species by comparing ] and a surface protein gene from various species, finding the human parasite ''P. falciparum'' to be more closely related to avian parasites than to other parasites of primates.<ref name=Perkins2014/> However, later studies sampling more ''Plasmodium'' species found the parasites of mammals to form a clade along with the genus '']'', while the parasites of birds or lizards appear to form a separate clade with evolutionary relationships not following the subgenera:<ref name=Perkins2014/><ref>{{cite journal|doi=10.1016/j.ympev.2007.11.012 |pmid=18248741 |title=A three-genome phylogeny of malaria parasites (''Plasmodium'' and closely related genera): Evolution of life-history traits and host switches |vauthors=Martinsen ES, Perkins SL, Schall JJ |journal=Molecular Phylogenetics and Evolution |volume=47 |issue=1 |date=April 2008 |pages=261–273}}</ref> | |||
| *Family '']''</br> | |||
| *Genus '']''</br> | |||
| *Subgenus '']''</br> | |||
| *Subgenus '']''</br> | |||
| {{clade| style=font-size:85%;line-height:85% | |||
| |1='']'' | |||
| |2={{clade | |||
| |1='']'' | |||
| |label2=''Plasmodium'' | |||
| |2= {{clade | |||
| |1=''Plasmodium'' of lizards and birds | |||
| |2= {{clade | |||
| |1=Subgenus ''Laverania'' | |||
| |2={{clade | |||
| |1=Subgenus ''Plasmodium'' | |||
| |2=Subgenus ''Vinckeia'' | |||
| |3='']'' (parasites of bats) | |||
| }} | |||
| }} | |||
| }} | |||
| }} | |||
| }} | |||
| Estimates for when different ''Plasmodium'' lineages diverged have differed broadly. Estimates for the diversification of the order Haemosporida range from around 16.2 million to 100 million years ago.<ref name=Perkins2014/> There has been particular interest in dating the divergence of the human parasite ''P. falciparum'' from other ''Plasmodium'' lineages due to its medical importance. For this, estimated dates range from 110,000 to 2.5 million years ago.<ref name=Perkins2014/> | |||
| Two other subgenera have been described in ''Plasmodium'' but are no longer used: '']'' and '']'' | |||
| == |
==Distribution== | ||
| ''Plasmodium'' species are distributed globally. All ''Plasmodium'' species are parasitic and must pass between a vertebrate host and an insect host to complete their life cycles. Different species of ''Plasmodium'' display different host ranges, with some species restricted to a single vertebrate and insect host, while other species can infect several species of vertebrates and/or insects. | |||
| === Vertebrates === | |||
| The full taxonomic name of a species includes the subgenus but this is often omitted. The full name indicates some features of the morphology and type of host species. The only two species in the sub genus ''Laverania'' are ''P. falciparum'' and ''P. reichenowi''. Species infecting ]s, and ] (the higher ]) with the exceptions of ''P. falciparum'' and ''P. reichenowi'' are classified in the subgenus ''Plasmodium''. Parasites infecting other ]s including lower primates (]s and others) are classified in the subgenus ''Vinckeia''. | |||
| ] | |||
| ''Plasmodium'' parasites have been described in a broad array of vertebrate hosts including reptiles, birds, and mammals.<ref name=Manguin/> While many species can infect more than one vertebrate host, they are generally specific to one of these ] (such as birds).<ref name=Manguin>{{cite book|title=Biodiversity of Malaria in the world |date=2008 |author1=Manguin, S. |author2=Carnevale, P. |author3=Mouchet, J. |author4=Coosemans, M. |author5=Julvez, J. |author6=Richard-Lenoble, D. |author7=Sircoulon, J. |publisher=John Libbey |isbn=978-2-7420-0616-8 |pages=13–15 |url=https://books.google.com/books?id=Sk0JBAAAQBAJ&q=malaria+reptile&pg=PA13 |access-date=15 March 2018}}</ref> | |||
| The distinction between ''P. falciparum'' and ''P. reichenowi'' and the other species infecting higer primates was based on the morphological findings but have since been confirmed by DNA analysis. ''Vinckeia'' while previously considered to be something of a taxanomic 'rag bag' has been recently shown - perhaps rather suprisingly - to form a coherent grouping. The remaining groupings here are based on the morphology of the parasites. Revisions to this system are likely to occur in the future as more species are subject to analysis of thier ]. | |||
| Humans are primarily infected by ] of ''Plasmodium'', with the overwhelming majority of severe disease and death caused by '']''.<ref name=Scully>{{cite journal |doi=10.1016/j.mib.2017.10.006|pmid=29096194|pmc=5733638 |title=Molecular interactions governing host-specificity of blood stage malaria parasites|journal=Current Opinion in Microbiology |volume=40|pages=21–31 |year=2017 |last1=Scully |first1=Erik J. |last2=Kanjee|first2=Usheer|last3=Duraisingh|first3=Manoj T.}}</ref> Some species that infect humans can also infect other primates, and zoonoses of certain species (e.g. '']'') from other primates to humans are common.<ref name=Scully/> Non-human primates also contain a ] that do not generally infect humans. Some of these can cause severe disease in primates, while others can remain in the host for prolonged periods without causing disease.<ref name=Nunn>{{cite book |author1=Nunn, C. |author2=Altizer, S. |date=2006 |title=Infectious Diseases in Primates: Behavior, Ecology and Evolution |url=https://books.google.com/books?id=1s0TDAAAQBAJ&q=plasmodium&pg=PA254 |edition=1st |publisher=Oxford University Press |pages=253–254 |isbn=978-0198565840 |access-date=16 March 2018}}</ref> Many other mammals also carry ], such as a variety of ], ], and ]s. Again, some species of ''Plasmodium'' can cause severe disease in some of these hosts, while many appear not to.<ref>{{cite journal|title=The rediscovery of malaria parasites of ungulates |journal=Parasitology |volume=143 |issue=12 |date=2016 |pages=1501–1508 |vauthors=Templeton TJ, Martinsen E, Kaewthamasorn M, Kaneko O |doi=10.1017/S0031182016001141|pmid=27444556 |s2cid=22397021 }}</ref> | |||
| ] infect a broad variety of birds. In general each species of ''Plasmodium'' infects one to a few species of birds.<ref name=Valkiunas2>{{cite book|title=Avian Malaria Parasites and Other Haemosporidia |author=Valkiunas, Gediminas |publisher=CRC Press |isbn=9780415300971 |date=2004 |chapter=Specificity and general Principles of Species Identification |pages=67–81}}</ref> ''Plasmodium'' parasites that infect birds tend to persist in a given host for years or for the life time of the host, although in some cases ''Plasmodium'' infections can result in severe illness and rapid death.<ref name=Valkiunas>{{cite book|title=Avian Malaria Parasites and Other Haemosporidia |author=Valkiunas, Gediminas |publisher=CRC Press |isbn=9780415300971 |date=2004 |chapter=General Section - Life Cycle and Morphology of Plasmodiidae Species |pages=27–35}}</ref><ref name=Valkiunas3>{{cite book|title=Avian Malaria Parasites and Other Haemosporidia |author=Valkiunas, Gediminas |publisher=CRC Press |isbn=9780415300971 |date=2004 |chapter=Pathogenicity |pages=83–111}}</ref> Unlike with ''Plasmodium'' species infecting mammals, those infecting birds are distributed across the globe.<ref name=Valkiunas2/> | |||
| Examples of the use of full names include: | |||
| ] of ''Plasmodium'' infect diverse ]. ''Plasmodium'' parasites have been described in most lizard ] and, like avian parasites, are spread worldwide.<ref name=Zug>{{cite book|title=Herpetology: An Introductory Biology of Amphibians and Reptiles |editor1=Zug, G. R. |editor2=Vitt, L. J. |isbn=978-0127826202 |publisher=Academic Press |date=2012 |url=https://books.google.com/books?id=nqgpcru2sfwC&q=malaria+reptile&pg=PA152 |access-date=16 March 2018 |page=152}}</ref> Again, parasites can result either in severe disease or be apparently asymptomatic depending on the parasite and the host.<ref name=Zug/> | |||
| ''Plasmodium (Asiamoeba) draconis''</br> | |||
| ''Plasmodium (Asiamoeba) vastator''</br> | |||
| A number of ] have been developed over the years to control ''Plasmodium'' infection in vertebrate hosts, particularly in humans. ] was used as a frontline antimalarial from the 17th century until widespread ] emerged in the early 20th century.<ref name="Blasco2018">{{cite journal |last1=Blasco |first1=Benjamin |last2=Leroy |first2=Didier |last3=Fidock |first3=David A. |author-link3=David A. Fidock |year=2017 |title=Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic |journal=Nature Medicine |volume=23 |issue=8 |pages=917–928 |doi=10.1038/nm.4381 |pmc=5747363 |pmid=28777791}}</ref> Resistance to quinine spurred the development of a broad array of antimalarial medications through the 20th century including ], ], ], ], ], and ].<ref name=Blasco2018/> In all cases, parasites resistant to a given drug have emerged within a few decades of the drugs deployment.<ref name=Blasco2018/> To combat this, antimalarial drugs are frequently used in combination, with ] currently the gold standard for treatment.<ref name=Cowman2016>{{cite journal |doi=10.1016/j.cell.2016.07.055 |pmid=27768886|title=Malaria: Biology and Disease |journal=Cell |volume=167|issue=3 |pages=610–624|year=2016|last1=Cowman |first1=Alan F|last2=Healer|first2=Julie |last3=Marapana |first3=Danushka|last4=Marsh |first4=Kevin|doi-access=free}}</ref> In general, antimalarial drugs target the life stages of ''Plasmodium'' parasites that reside within vertebrate red blood cells, as these are the stages that tend to cause disease.<ref name=Haldar2018>{{cite journal|doi=10.1038/nrmicro.2017.161 |pmid=29355852|pmc=6371404|title=Drug resistance in Plasmodium |journal=Nature Reviews Microbiology|volume=16|issue=3 |pages=156–170 |year=2018|last1=Haldar |first1=Kasturi |last2=Bhattacharjee|first2=Souvik|last3=Safeukui|first3=Innocent}}</ref> However, drugs targeting other stages of the parasite life cycle are under development in order to prevent infection in travelers and to prevent transmission of sexual stages to insect hosts.<ref>{{cite journal|doi=10.1002/med.21486 |pmid=29372568 |title=Multistage inhibitors of the malaria parasite: Emerging hope for chemoprotection and malaria eradication |journal=Medicinal Research Reviews |volume=38 |issue=5 |pages=1511–1535 |year=2018 |last1=Poonam |last2=Gupta |first2=Yash |last3=Gupta |first3=Nikesh |last4=Singh |first4=Snigdha |last5=Wu |first5=Lidong |last6=Chhikara |first6=Bhupender Singh |last7=Rawat |first7=Manmeet |last8=Rathi |first8=Brijesh |s2cid=25711437 }}</ref> | |||
| ''Plasmodium (Carinamoeba) basilisci''</br> | |||
| ''Plasmodium (Carinamoeba) minasense''</br> | |||
| ''Plasmodium (Carinamoeba) rhadinurum''</br> | |||
| ''Plasmodium (Carinamoeba) volans''</br> | |||
| <gallery widths="200px" heights="160px"> | |||
| ''Plasmodium (Giovannolaia) circumflexum''</br> | |||
| File:Saving_Lives_with_SMS_for_Life.jpg|A clinic for treating human malaria in Tanzania | |||
| ''Plasmodium (Giovannolaia) dissanaikei''</br> | |||
| File:Anolis carolinensis.jpg|Over 3000 species of lizard, including the ] (''Anolis carolinensis''), carry some 90 kinds of malaria. | |||
| ''Plasmodium (Giovannolaia) durae''</br> | |||
| </gallery> | |||
| ''Plasmodium (Giovannolaia) fallax''</br> | |||
| ''Plasmodium (Giovannolaia) garnhami''</br> | |||
| ''Plasmodium (Giovannolaia) gundersi''</br> | |||
| ''Plasmodium (Giovannolaia) lophurae''</br> | |||
| ''Plasmodium (Giovannolaia) pedioecetii''</br> | |||
| ''Plasmodium (Giovannolaia) polare''</br> | |||
| === Insects === | |||
| ''Plasmodium (Haemamoeba) cathemerium''</br> | |||
| ]'' is among the blood-feeding insects that can be infected by a species of ''Plasmodium''.]] | |||
| ''Plasmodium (Haemamoeba) coggeshalli''</br> | |||
| ''Plasmodium (Haemamoeba)elongatum'' </br> | |||
| ''Plasmodium (Haemamoeba) gallinacium''</br> | |||
| ''Plasmodium (Haemamoeba) giovannolai''</br> | |||
| ''Plasmodium (Haemamoeba) matutinum''</br> | |||
| ''Plasmodium (Haemamoeba) parvulum''</br> | |||
| ''Plasmodium (Haemamoeba) relictum''</br> | |||
| ''Plasmodium (Haemamoeba) subpraecox''</br> | |||
| ''Plasmodium (Haemamoeba) tejera''</br> | |||
| In addition to a vertebrate host, all ''Plasmodium'' species also infect a ] insect host, generally a mosquito (although some reptile-infecting parasites are transmitted by ]). Mosquitoes of the genera '']'', '']'', '']'', '']'' and '']'' act as insect hosts for various ''Plasmodium'' species. The best studied of these are the ''Anopheles'' mosquitoes which host the ''Plasmodium'' parasites of human malaria, as well as ''Culex'' mosquitoes which host the ''Plasmodium'' species that cause malaria in birds. Only female mosquitoes are infected with ''Plasmodium'', since only they feed on the blood of vertebrate hosts.<ref>{{Cite journal |last1=Crompton |first1=Peter D. |last2=Moebius |first2=Jacqueline |last3=Portugal |first3=Silvia |last4=Waisberg |first4=Michael |last5=Hart |first5=Geoffrey |last6=Garver |first6=Lindsey S. |last7=Miller |first7=Louis H. |last8=Barillas-Mury |first8=Carolina |last9=Pierce |first9=Susan K. |date=2014 |title=Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease |journal=Annual Review of Immunology |volume=32 |issue=1 |pages=157–187 |doi=10.1146/annurev-immunol-032713-120220 |pmid=24655294 |pmc=4075043}}</ref> Different species affect their insect hosts differently. Sometimes, insects infected with ''Plasmodium'' have reduced lifespan and reduced ability to produce offspring.<ref name=Busula>{{cite journal |doi=10.1016/j.pt.2017.08.010 |pmid=28942108 |title=Mechanisms of Plasmodium -Enhanced Attraction of Mosquito Vectors |journal=Trends in Parasitology |volume=33 |issue=12 |pages=961–973 |year=2017 |last1=Busula |first1=Annette O. |last2=Verhulst |first2=Niels O. |last3=Bousema |first3=Teun |last4=Takken |first4=Willem |last5=De Boer |first5=Jetske G.}}</ref> Further, some species of ''Plasmodium'' appear to cause insects to prefer to bite infected vertebrate hosts over non-infected hosts.<ref name=Busula/><ref>{{cite journal |doi=10.1016/j.cois.2017.02.002 |pmid=28602239 |title=Effects of malaria infection on mosquito olfaction and behavior: Extrapolating data to the field |journal=Current Opinion in Insect Science |volume=20 |pages=7–12 |year=2017 |last1=Stanczyk |first1=Nina M. |last2=Mescher |first2=Mark C. |last3=De Moraes |first3=Consuelo M.|doi-access=free }}</ref><ref>{{cite journal |doi=10.1101/cshperspect.a025593 |pmid=28389513 |title=Anopheline Reproductive Biology: Impacts on Vectorial Capacity and Potential Avenues for Malaria Control |journal=Cold Spring Harbor Perspectives in Medicine |volume=7 |issue=12 |pages=a025593 |year=2017 |last1=Mitchell |first1=Sara N. |last2=Catteruccia |first2=Flaminia|pmc=5710097 |author-link2=Flaminia Catteruccia}}</ref> | |||
| ''Plasmodium (Huffia) elongatum''</br> | |||
| ''Plasmodium (Huffia) hermani''</br> | |||
| == History == | |||
| ''Plasmodium (Laverania) falciparum''</br> | |||
| ] first described parasites in the blood of malaria patients in 1880.<ref name=CDCHistory>{{cite web|url=https://www.cdc.gov/malaria/about/history/index.html |access-date=31 May 2016 |title=The History of Malaria, an Ancient Disease |publisher=U.S. Centers for Disease Control and Prevention}}</ref> He named the parasite ''Oscillaria malariae''.<ref name=CDCHistory/> In 1885, zoologists ] and ] reexamined the parasite and termed it a member of a new genus, ''Plasmodium'', named for the resemblance to the ] of ] of the same name.<ref name=McFadden2012>{{cite journal | last1 = McFadden | first1 = G. I. | year = 2012 | title = Plasmodia – don't | journal = Trends Parasitol | volume = 28 | issue = 8 | pages = 306 | doi=10.1016/j.pt.2012.05.006 | pmid = 22738856}}</ref>{{refn|group=notes|The plural of ''Plasmodium'' is not ''Plasmodia''. Instead multiple species of the genus are referred to as "''Plasmodium'' species".<ref name=McFadden2012/>}} The fact that several species may be involved in causing different forms of malaria was first recognized by ] in 1886.<ref name=CDCHistory/> Soon thereafter, ] and ] named the parasites causing two different types of human malaria '']'' and '']''.<ref name=CDCHistory/> In 1897, ] identified and named '']''. This was followed by the recognition of the other two species of ''Plasmodium'' which infect humans: '']'' (1922) and '']'' (identified in ]s in 1931; in humans in 1965).<ref name=CDCHistory/> The contribution of insect hosts to the ''Plasmodium'' life cycle was described in 1897 by ] and in 1899 by Giovanni Batista Grassi, ] and ].<ref name=CDCHistory/> | |||
| ''Plasmodium (Laverania) reichenowi''</br> | |||
| In 1966, ] proposed separating ''Plasmodium'' into nine subgenera based on host specificity and parasite morphology.<ref name=Martinsen2013/> This included four subgenera that had previously been proposed for bird-infecting ''Plasmodium'' species by A. Corradetti in 1963.<ref name="Corradetti1963">{{cite journal |author1=Corradetti A. |author2=Garnham P.C.C. |author3=Laird M. |title=New classification of the avian malaria parasites |journal=Parassitologia |volume=5 |pages=1–4 |year=1963 }}</ref><ref name=Valkiunas4/> This scheme was expanded upon by Sam R. Telford in 1988 when he reclassified ''Plasmodium'' parasites that infect reptiles, adding five subgenera.<ref name="Telford1988">{{cite journal |author=Telford S |title=A contribution to the systematics of the reptilian malaria parasites, family Plasmodiidae (Apicomplexa: Haemosporina) |journal=Bulletin of the Florida State Museum Biological Sciences |volume=34 |issue=2 |pages=65–96 |year=1988 |url=http://ufdcweb1.uflib.ufl.edu/UF00095820/00001/3 |access-date=2014-03-25 |archive-date=2018-09-26 |archive-url=https://web.archive.org/web/20180926161500/http://ufdcweb1.uflib.ufl.edu/UF00095820/00001/3 |url-status=dead }}</ref><ref name=Martinsen2013/> In 1997, G. Valkiunas reclassified the bird-infecting ''Plasmodium'' species adding a fifth subgenus: ''Bennettinia''.<ref name=Valkiunas4/><ref name="Valkiunas1997">{{cite journal |author=Valkiunas, G. |title=Bird Haemosporidia |journal=Acta Zoologica Lituanica |volume=3–5 |pages=1–607 |year=1997 |issn=1392-1657 }}</ref> | |||
| ''Plasmodium (Novyella) bambusicolai''</br> | |||
| ''Plasmodium (Novyella) corradettii''</br> | |||
| ''Plasmodium (Novyella) hexamerium''</br> | |||
| ''Plasmodium (Novyella) juxtanucleare''</br> | |||
| ''Plasmodium (Novyella) kempi''</br> | |||
| ''Plasmodium (Novyella) nucleophilum''</br> | |||
| ''Plasmodium (Novyella) papernai''</br> | |||
| ''Plasmodium (Novyella) rouxi''</br> | |||
| ''Plasmodium (Novyella) vaughani''</br> | |||
| ==See also== | |||
| ''Plasmodium (Paraplasmodium) mexicanum''</br> | |||
| * ] | |||
| ''Plasmodium (Paraplasmodium) chiricahuae''</br> | |||
| * ] | |||
| * ] | |||
| == Notes== | |||
| ''Plasmodium (Plasmodium) brasilianum''<br/> | |||
| {{Reflist|group=notes}} | |||
| ''Plasmodium (Plasmodium) cynomolgi''<br/> | |||
| ''Plasmodium (Plasmodium) eylesi''</br> | |||
| ''Plasmodium (Plasmodium) fieldi''</br> | |||
| ''Plasmodium (Plasmodium) fragile''<br/> | |||
| ''Plasmodium (Plasmodium) georgesi''</br> | |||
| ''Plasmodium (Plasmodium) girardi''</br> | |||
| ''Plasmodium (Plasmodium) gonderi''</br> | |||
| ''Plasmodium (Plasmodium) inui''<br/> | |||
| ''Plasmodium (Plasmodium) jefferyi''</br> | |||
| ''Plasmodium (Plasmodium) knowlei''</br> | |||
| ''Plasmodium (Plasmodium) hyobati''<br/> | |||
| ''Plasmodium (Plasmodium) malariae''</br> | |||
| ''Plasmodium (Plasmodium) ovale''<br/> | |||
| ''Plasmodium (Plasmodium) petersi''</br> | |||
| ''Plasmodium (Plasmodium) pitheci''</br> | |||
| ''Plasmodium (Plasmodium) rhodiani''</br> | |||
| ''Plasmodium (Plasmodium) schweitzi''</br> | |||
| ''Plasmodium (Plasmodium) semiovale''<br/> | |||
| ''Plasmodium (Plasmodium) shortii''</br> | |||
| ''Plasmodium (Plasmodium) silvaticum''</br> | |||
| ''Plasmodium (Plasmodium) simium''<br/> | |||
| ''Plasmodium (Plasmodium) vivax''<br/> | |||
| ''Plasmodium (Plasmodium) youngi''</br> | |||
| == References == | |||
| ''Plasmodium (Sauramoeba) achiotense''</br> | |||
| {{Reflist}} | |||
| ''Plasmodium (Sauramoeba) aeuminatum''</br> | |||
| ''Plasmodium (Sauramoeba) agamae''</br> | |||
| ''Plasmodium (Sauramoeba) giganteum''</br> | |||
| ''Plasmodium (Sauramoeba) heischi''</br> | |||
| ''Plasmodium (Sauramoeba) josephinae''</br> | |||
| ''Plasmodium (Sauramoeba) pelaezi''</br> | |||
| ''Plasmodium (Sauramoeba) tropiduri''</br> | |||
| == Further reading == | |||
| ''Plasmodium (Vinckeia) aegyptensis''</br> | |||
| ===Identification=== | |||
| ''Plasmodium (Vinckeia) anomaluri''</br> | |||
| ''Plasmodium (Vinckeia) atheruri''<br/> | |||
| ''Plasmodium (Vinckeia) berghei''</br> | |||
| ''Plasmodium (Vinckeia) booliati''</br> | |||
| ''Plasmodium (Vinckeia) bubalis''</br> | |||
| ''Plasmodium (Vinckeia) bucki''<br/> | |||
| ''Plasmodium (Vinckeia) cephalophi''</br> | |||
| ''Plasmodium (Vinckeia) chabaudi''</br> | |||
| ''Plasmodium (Vinckeia) coulangesi''<br/> | |||
| ''Plasmodium (Vinckeia) cyclopsi''</br> | |||
| ''Plasmodium (Vinckeia) foleyi''<br/> | |||
| ''Plasmodium (Vinckeia) girardi''<br/> | |||
| ''Plasmodium (Vinckeia) inopinatum''</br> | |||
| ''Plasmodium (Vinckeia) lemuris''<br/> | |||
| ''Plasmodium (Vinckeia) odocoilei''</br> | |||
| ''Plasmodium (Vinckeia) percygarnhami''<br/> | |||
| ''Plasmodium (Vinckeia) sandoshami''<br/> | |||
| ''Plasmodium (Vinckeia) traguli''</br> | |||
| ''Plasmodium (Vinckeia) uilenbergi''<br/> | |||
| ''Plasmodium (Vinckeia) vinckei''</br> | |||
| ''Plasmodium (Vinckeia) watteni''</br> | |||
| ''Plasmodium (Vinckeia) yoelli''</br> | |||
| * {{cite book |last=Garnham |first=P. C. |title=Malaria Parasites And Other Haemosporidia |publisher=Blackwell |location=Oxford |year=1966 |isbn=978-0397601325}} | |||
| * {{cite book |last=Valkiunas |first=Gediminas |title=Avian Malaria Parasites and Other Haemosporidia |publisher=CRC Press |location=Boca Raton |year=2005 |isbn=9780415300971}} | |||
| ===Biology=== | |||
| * Notes | |||
| * {{cite journal |author1=Baldacci, P. |author2=Ménard, R. |title=The elusive malaria sporozoite in the mammalian host |journal=Mol. Microbiol. |volume=54 |issue=2 |pages=298–306 |date=October 2004 |pmid=15469504 |doi=10.1111/j.1365-2958.2004.04275.x |s2cid=30488807 |doi-access=free }} | |||
| * {{cite journal |author=Bledsoe, G. H. |title=Malaria primer for clinicians in the United States |journal=South. Med. J. |volume=98 |issue=12 |pages=1197–204; quiz 1205, 1230 |date=December 2005 |pmid=16440920 |url=http://www.sma.org/pdfs/objecttypes/smj/91C48D32-BCD4-FF25-565C69314AF7EB48/1196.pdf |doi=10.1097/01.smj.0000189904.50838.eb |s2cid=30660702 |url-status=dead |archive-url=https://web.archive.org/web/20090326131858/http://www.sma.org/pdfs/objecttypes/smj/91C48D32-BCD4-FF25-565C69314AF7EB48/1196.pdf |archive-date=2009-03-26 }} | |||
| * {{cite journal |author=Shortt, H. E. |title=Life-cycle of the mammalian malaria parasite |journal=Br. Med. Bull. |volume=8 |issue=1 |pages=7–9 |year=1951 |pmid=14944807 |doi=10.1093/oxfordjournals.bmb.a074057 }} | |||
| ===History=== | |||
| * {{cite journal |author=Slater, L. B. |title=Malarial birds: modeling infectious human disease in animals |journal=Bull Hist Med |volume=79 |issue=2 |pages=261–94 |year=2005 |pmid=15965289 |doi=10.1353/bhm.2005.0092 |s2cid=23594155 }} | |||
| ==External links== | |||
| Species of Novyella are characterized by very small schizonts. Sexual stages in this subgenus resemble those of ''Haemoproteus''. | |||
| {{Wikispecies|Plasmodium}} | |||
| {{Commons category}} | |||
| * | |||
| * | |||
| {{Malaria}} | |||
| == Infections in primates == | |||
| {{Taxonbar|from=Q130948}} | |||
| {{Authority control}} | |||
| ] | |||
| The species that infect primates other than humans include: ''P. brasilianum'', ''P. bucki'', ''P. coulangesi'', ''P. cynomolgi'', ''P. coatneyi'', ''P. eylesi'', ''P. fieldi'', ''P. foleyi'', ''P. fragile'', ''P. girardi'', ''P. georgesi'', ''P. gonderi'', ''P. hylobati'', ''P. inui'', ''P. knowlesi'', ''P. lemuris'', ''P. percygarnhami'', ''P. petersi'', ''P. reichenowi'', ''P. rodhaini'', ''P. sandoshami'', ''P shortii'', ''P. silvaticum'', ''P. simiovale'', ''P. simium'' and ''P. uilenbergi''. | |||
| ] | |||
| * Host record | |||
| Most if not all ''Plasmodium'' species infect more than one host: the host records shown here should be regarded as being incomplete. | |||
| ''P. bucki'' - '']'' | |||
| ''P. coatneyi'' - several ] species: '']'' and '']''. | |||
| ''P. coulangesi'' - '']'' | |||
| ''P. cynomolgi'' - '']'', '']'', '']'', '']'', '']'', '']'' and '']'' | |||
| ''P. eylesi'' - several ] ('']'') species | |||
| ''P. fieldi'' - '']'' and '']'' | |||
| ''P. foleyi'' - '']'' | |||
| ''P. fragile'' - several ] species - '']'', '']'', '']'', and '']'' | |||
| ''P. georgesi'' - '']'' | |||
| ''P. girardi'' - '']'' | |||
| ''P. gonderi'' - ] ('']'') and ]s ('']'') | |||
| ''P. hylobati'' - several ] ('']'') species | |||
| ''P. inui'' - the ] black ] ('']''), '']'', '']'', and several '']'' species | |||
| ''P. jefferyi'' - several ] ('']'') species | |||
| ''P. knowlesi'' - '']'', '']'', and '']'' | |||
| ''P. knowlesi edesoni'' - the ]nese long-tailed ] ('']'') | |||
| ''P. lemuris'' - the ] '']'' | |||
| ''P. percygarnhami'' - '']'' | |||
| ''P. petersi'' - '']'' | |||
| ''P. pitheci'' - ]s ('']'') | |||
| ''P. reichenowi'' - chimpanzees ('']'') species and gorillas ('']'') species | |||
| ''P. rodhaini'' - chimpanzees ('']'') species and gorillas ('']'') species | |||
| ''P. sandoshami'' - the ]n flying ] ('']'') | |||
| ''P. schwetzi'' - chimpanzees ('']'') species and gorillas ('']'') species | |||
| ''P. semiovale'' - '']'' | |||
| ''P. silvaticum'' - ]s ('']'') | |||
| ''P. simium'' - the ] ('']'') and several ] ('']'') species | |||
| ''P. shortii'' - '']'', and '']'' | |||
| ''P. uilenbergi'' - '']'' | |||
| ''P. youngei'' - white handed ] ('']'') | |||
| * Mosquito vectors | |||
| '']'' - ''P. inui'' | |||
| '']'' - ''P. coatneyi'' and ''P. vivax'' <ref name = Collins2002>Collins WE,Sullivan JS,Nace D, Williams T,Sullivan JJ, Galland GG,Grady KK and Bounngaseng A. 2002. Experimental infection of ''Anopheles farauti'' with different species of ''Plasmodium''. J. Parasitol. 88(2):295-298. </ref> | |||
| '']'' - ''P. youngei'' | |||
| '']'' - ''P. cynomogli'' and ''P. inui'' | |||
| '']'' - ''P. youngei'' | |||
| *Subspecies | |||
| ''P. cynomolgi'' - ''P. cynomolgi bastianelli'' and ''P. cynomolgi ceylonensis''. | |||
| ''P. inui'' - ''P. inui inui'' and ''P. inui shortii'' | |||
| ''P. knowlesi'' - ''P. knowlesi edesoni'' and ''P. knowlesi knowlesi''. | |||
| ''P. vivax'' - ''P. vivax hibernans'', ''P. vivax chesson'' and ''P. vivax multinucleatum''. | |||
| * Interrelatedness | |||
| The evolution of these species is still being worked out and the relationships given here should be regarded as tentative. | |||
| ''P. brasilianum'', ''P. inui'' and ''P. rodhaini'' are similar to ''P. malariae'' | |||
| ''P. cynomolgi'', ''P. fragile'', ''P. knowlesi'', ''P. simium'' and ''P. schwetzi'' are similar to ''P. vivax'' | |||
| ''P. fieldi'' and ''P. simiovale'' are similar to ''P. ovale'' | |||
| ''P. falciparum'' is closely related to ''P. reichenowi''. | |||
| This last grouping while originally made on morphological grounds now has considerable support at the ] level. | |||
| *Notes | |||
| ''P. kochi'' has been described as a parasite of monkeys. This species is currently classified as ''Hepatocystis kochi''. This may be subject to revision. | |||
| ''P. brasilianum'' and ''P. rodhaini'' seem likely to be the same species as ''P. malariae.'' | |||
| == Infections in non primate mammals == | |||
| The subgenus Vinckeia was created by ] to accommodate the mammalian parasites other than those infecting primates. Species infecting lemurs have also been included in this subgenus. | |||
| ''P. aegyptensis'', ''P. bergei'', ''P. chabaudi'', ''P. inopinatum'', ''P. yoelli'' and ''P. vinckei'' infect ]. ''P. bergei'', ''P. chabaudi'', ''P. yoelli'' and ''P. vinckei'' have been used to study malarial infections in the ]. Other members of this subgenus infect other ]ian ]s. | |||
| * Host records | |||
| ''P. aegyptensis'' - ]ian grass rat ('']'')<ref name=Abd-el-Aziz1975> Abd-el-Aziz GA, Landau I, and Miltgen F. (1975) Description of Plasmodium aegyptensis n. sp., presumed parasite of the Muridae Arvicanthis noloticus in Upper Egypt Ann Parasitol Hum Comp. 50(4):419-424.</ref> | |||
| ''P. atheruri'' - ]n ] ('']''), large vesper ] ('']'') and '']'' | |||
| ''P. booliati'' - ] <ref name=Sandosham1965> Sandosham AA, Yap LF, Omar I. (1965) A malaria parasite, plasmodium (Vinckeia) booliati sp.nov., from a Malayan giant flying squirrel. Med J Malaya. 20(1):3-7 </ref></br> | |||
| ''P. bubalis'' - ]es ('']'') | |||
| ''P. cephalophi'' - grey ] ('']'') <ref name="Keymer1966> Keymer IF. (1966) Studies on Plasmodium (Vinckeia) cephalophi of the grey duiker (Sylvicapra grimmia). Ann Trop Med Parasitol. 60(2):129-138 </ref></br> | |||
| ''P. cyclopsi'' - the ] ('']'') <ref name=Landau1978> Landau I, and Chabaud AG. (1978) Description of P. cyclopsi n. sp. a parasite of the microchiropteran bat Hipposideros cyclops in Gabon. Ann. Parasitol. Hum. Comp. 53(3):247-253.</ref> | |||
| ''P. odocoilei'' - ] ('']'') | |||
| ''P. traguli'' - the ] | |||
| ''P. watteni'' - ]n giant flying squirrel ('']'')<ref name="Lien1968> Lien JC, Cross JH. (1968) Plasmodium (Vinckeia) watteni sp. n. from the Formosan giant flying squirrel, Petaurista petaurista grandis. J Parasitol. 54(6):1171-1174 </ref></br> | |||
| * Vectors | |||
| '']'' - ''P. atheruri'' | |||
| * Subspecies | |||
| ''P. yoellii'' - ''P. yoelli nigeriensis'' and ''P. yoelli yoelli''. | |||
| ''P. vinkei'' - ''P. vinckei brucechwatti'', ''P. vinckei petteri'' and ''P. vinckei vinckei''. | |||
| ''P. chabaudi'' - ''P. chabaudi adami'' and ''P. chabaudi chabaudi'' | |||
| * Notes | |||
| ''Calomys callosus'' seems unlikely to be a natural host for ''P. atheruri'' as ''P. atheruri'' is found in ] and ''Calomys callosus'' in ]. | |||
| == Infections in birds == | |||
| Species in five of these subgenera infect ]s - Bennettinia, Giovannolaia, Haemamoeba, Huffia and Novyella. <ref name=Wiersch2005>Wiersch SC, Maier WA, Kampen H. Plasmodium (Haemamoeba) cathemerium gene sequences for phylogenetic analysis of malaria parasites. Parasitol Res. 96(2): 90-94 </ref> Giovannolaia appears to be a polyphytic group and may be sudivided in the future. <ref name=Martinsen2006> Martinsen ES,Waite JL,Schall JJ Morphologically defined subgenera of Plasmodium from avian hosts: test of monophyly by phylogenetic analysis of two mitochondrial genes (2006) Parasitology 1 - 8 </ref> | |||
| Species infecting birds include: ''P. anasum'', ''P. bambusicolai'', ''P. bigueti'', ''P. cathemerium'', ''P. circumflexum'', ''P. coggeshalli'', '' P. corradettii'', ''P. coturnix'', ''P. dissanaikei'', ''P. durae'', ''P. elongatum'', ''P. fallax'', ''P forresteri'', ''P. gallinacium'', ''P. garnhami'', ''P. giovannolai'', ''P. griffithsi'', ''P. gabaldoni'', ''P. gundersi'', ''P. hegneri'', ''P. hermani'', ''P. hexamerium'', ''P. huffi'', ''P. juxtanucleare'', ''P. kempi'', ''P. lophurae'', ''P. matutinum'', ''P. nucleophilum'', ''P. papernai'', ''P. paranucleophilum'', ''P. parvulum'', ''P. pediocetti'', ''P. pitmani'', ''P. pinotti'', ''P. polare'', ''P. praecox'', ''P. relictum'', ''P. rouxi'', ''P. tenue'', ''P. tejerai'' and ''P. vaughani''. | |||
| * Subspecies | |||
| ''P. relictum'' has been divded into two subspecies: ''P. relictum relictum'' and ''P. relictum capistranoae''. | |||
| * Notes | |||
| ''Plasmodium relictum'' is probably responsible for more bird ]ions than any other protist. | |||
| ''P. praecox'' has been re classiified as ''P. relictum''. Both names have been recorded here as a guide to the literature. | |||
| ''P. dominicana'' is species known only from fossil amber. <ref name=Poinar2005> Poinar, G. (2005) Plasmodium dominicana n. sp. (Plasmodiidae: Haemospororida) from Tertiary Dominican amber. Systematic Parasitology 61 (1) 47-52</ref> It is thought to have been a species infecting birds. | |||
| == Infections in reptiles == | |||
| Species in the subgenera Carinamoeba, Paraplasmodium and Sauramoeba infect ]. <ref name=Shall2000> Schall JJ (2000) Transmission success of the malaria parasite Plasmodium mexicanum into its vector: role of gametocyte density and sex ratio. Parasitology. 121 (6):575-580 </ref> | |||
| Species infecting reptiles include: ''P. achiotense'', ''P. aeuminatum'', ''P. agamae'', ''P. azurophilum'', ''P. balli'', ''P. basilisci'', ''P. brygooi'', ''P. chiricahuae'', ''P. circularis'', ''P. cnemidophori'', ''P. colombiense'', ''P. diminutivum'', ''P. diploglossi'', ''P. egerniae'', ''P. fairchildi'', ''P. floridense'', ''P. giganteum'', ''P. gracilis'', ''P. guyannense'', ''P. heischi'', ''P. holaspi'', ''P. icipeensis'', ''P. josephinae'', ''P. kentropyxi'', ''P. lepidoptiformis'', ''P. lygosomae'', ''P. mabuiae'', ''P. mackerrasae'', ''P. maculilabre'', ''P. mexicanum'', ''P. minasense'', ''P. pelaezi'', ''P. pifanoi'', ''P. pitmani'', ''P. rhadinurum'', ''P. robinsoni'', ''P. sasai'', ''P. tomodoni'', ''P. torrealbai'', ''P. tribolonoti'', ''P. tropiduri'', ''P. vacuolatum'', ''P. volans'' and ''P. wenyoni''. | |||
| * Host records | |||
| ''P. heischi'' - ] (]) <ref name= Granham1984> Garnham PC, Telford SR Jr. (1984) A new malaria parasite Plasmodium (Sauramoeba) heischi in skinks (Mabuya striata) from Nairobi, with a brief discussion of the distribution of malaria parasites in the family Scincidae. J Protozool. 31(4):518-521.</ref> | |||
| ''P. giganteum'' - ]. <ref name=Southgate1970> Southgate BA. (1970) Plasmodium (Sauramoeba) giganteum in Agama cyanogaster: a new host record. Trans R Soc Trop Med Hyg. 64(1):12-13 </ref> | |||
| ''P. siamense'' - lizards. <ref name=Telford1986> Telford SR (1986) Fallisia parasites (Haemosporidia: Plasmodiidae) from the flying lizard, Draco maculatus (Agamidae) in Thailand. J Parasitol. 72(5):766-769 </ref> | |||
| ''P. tribolonoti'' - ]s | |||
| ''P. gracilis'' - skinks | |||
| ''P. lygosomae'' - skink (]) | |||
| ''P. tomodoni'' - ]s | |||
| ''P. wenyoni'' - snakes | |||
| * Vectors | |||
| ''P. agamae'' - '']'' or '']'' species | |||
| * Subspecies | |||
| ''P. minasense'' - ''P. minasense minasense'', ''P. minasense carinii'', ''P. minasense anolisi'', ''P. minasense capitoi'', ''P. minasense plicae'', ''P. minasense tegui'' and ''P. minasense diminutivum''. <ref name= Telford1979> Telford SR Jr. (1979) A taxonomic revision of small neotropical saurian Malarias allied to Plasmodium minasense. Ann Parasitol Hum Comp. 54(4):409-422</ref> An additional subspecies ''P. minasense calcaratae'' has also been decribed. <ref name=Telford2003>Telford SR Jr and Telford SR 3rd. Rediscovery and redescription of Plasmodium pifanoi and description of two additional Plasmodium parasites of Venezuelan lizards. Journal of Parasitology (2003) 89(2):362-368</ref> | |||
| ''P. traguli'' - ''P. traguli traguli'' and ''P. traguli memmina''. | |||
| ''P. tropiduri'' - ''P. tropiduri tropiduri'', ''P. tropiduri panamense'' and ''P. tropiduri aquaticum''. <ref name=Telford1979> Telford SR Jr.A taxonomic reconsideration of some Plasmodium species from iguanid lizards. Ann Parasitol Hum Comp. (1979) 54(2):129-144 </ref> | |||
| ''P. lygosomae'' - ''P. lygosomae nucleoversans''. | |||
| == Life cycle == | |||
| The life cycle of ''Plasmodium'' is very complex. ]s from the saliva of a biting female mosquito are transmitted to either the blood or the lymphatic system<ref name="Lymph">]'', '']'', '']'', '']'' and '']'' may act as vectors. The currently known vectors for human malaria (> 100 species) all belong to the genus ''Anopheles''. The life cycle of ''Plasmodium'' was discovered by Ross who worked with species from the genus ''Culex''. | |||
| The sporozoites migrate to the ] and invade hepatocytes. The so-called latent or dormant stage of the ''Plasmodium'' sporozoite in the liver is called the hypnozoite. From the hepatocytes, the parasite replicates into thousands of ]s, which then invade ]s. Here the parasite grows from a ring-shaped form to a larger ] form. In the ] stage, the parasite divides several times to produce new merozoites, which leave the red blood cells and travel within the bloodstream to invade new red blood cells. Most merozoites continue this replicative cycle, but some merozoites differentiate into male or female sexual forms (]s) (also in the blood), which are taken up by the female ''Anopheles'' mosquito. In the mosquito's midgut, the ]s develop into ]s and ] each other, forming motile ]s called ]. The ookinetes penetrate and escape the midgut, then embed themselves onto the exterior of the gut membrane. Here they divide many times to produce large numbers of tiny elongated ]s. These sporozoites migrate to the salivary glands of the mosquito where they are injected into the blood of the next host the mosquito bites. The sporozoites move to the liver where they repeat the cycle. | |||
| This life cycle is best understood in terms of its ]. It is thought that ''Plasmodium'' evolved from a parasite spread by the orofaecal route which infected the intestinal wall. At some point this parasite evolved the ability to infect the liver. This pattern is seen in the genus '']'' to which Plasmodium is distantly related. | |||
| At some later point this ancestor developed the ability to infect ] and to survive and infect ]es. Once mosquito transmission was firmly established the previous orofecal route of transmission was lost. | |||
| == Molecular biology == | |||
| On a molecular level, the parasite damages red blood cells using ] enzymes. Plasmepsins are ] which degrade ]. | |||
| ==Notes== | |||
| <!--<nowiki> | |||
| See http://en.wikipedia.org/Wikipedia:Footnotes for an explanation of how to generate footnotes using the <ref> and </ref> tags, and the template below. | |||
| </nowiki>--> | |||
| {{FootnotesSmall|resize=100%}} | |||
| ==References== | |||
| * Short, H. E. (1951) ''Life-cycle of the mammalian malaria parasite" ''British Medical Bulletin'' 8(1): pp. 7-9, (PMID 14944807); | |||
| * Baldacci, Patricia and Ménard, Robert (Oct. 2004) "The elusive malaria sporozoite in the mammalian host" ''Molecular Microbiology'' 54(2): pp. 298-306, (AN 14621725); | |||
| * (PMID 16440920); | |||
| ==External links== | |||
| * (Flash animations) | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 06:32, 20 August 2024
Genus of parasitic protists that can cause malaria For the multinucleate stage of some microorganisms, see Plasmodium (life cycle).
| Plasmodium | |
|---|---|

| |
| False-colored electron micrograph of a sporozoite | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemospororida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium Marchiafava & Celli, 1885 |
Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue (often the liver) before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect (mosquitoes in majority cases), continuing the life cycle.
Plasmodium is a member of the phylum Apicomplexa, a large group of parasitic eukaryotes. Within Apicomplexa, Plasmodium is in the order Haemosporida and family Plasmodiidae. Over 200 species of Plasmodium have been described, many of which have been subdivided into 14 subgenera based on parasite morphology and host range. Evolutionary relationships among different Plasmodium species do not always follow taxonomic boundaries; some species that are morphologically similar or infect the same host turn out to be distantly related.
Species of Plasmodium are distributed globally wherever suitable hosts are found. Insect hosts are most frequently mosquitoes of the genera Culex and Anopheles. Vertebrate hosts include reptiles, birds, and mammals. Plasmodium parasites were first identified in the late 19th century by Charles Laveran. Over the course of the 20th century, many other species were discovered in various hosts and classified, including five species that regularly infect humans: P. vivax, P. falciparum, P. malariae, P. ovale, and P. knowlesi. P. falciparum is by far the most lethal in humans, resulting in hundreds of thousands of deaths per year. A number of drugs have been developed to treat Plasmodium infection; however, the parasites have evolved resistance to each drug developed.
Although the parasite can also infect people via blood transfusion, this is very rare, and Plasmodium cannot be spread from person to person. Some of subspecies of Plasmodium are obligate intracellular parasites.
Description

The genus Plasmodium consists of all eukaryotes in the phylum Apicomplexa that both undergo the asexual replication process of merogony inside host red blood cells and produce the crystalline pigment hemozoin as a byproduct of digesting host hemoglobin. Plasmodium species contain many features that are common to other eukaryotes, and some that are unique to their phylum or genus. The Plasmodium genome is separated into 14 chromosomes contained in the nucleus. Plasmodium parasites maintain a single copy of their genome through much of the life cycle, doubling the genome only for a brief sexual exchange within the midgut of the insect host. Attached to the nucleus is the endoplasmic reticulum (ER), which functions similarly to the ER in other eukaryotes. Proteins are trafficked from the ER to the Golgi apparatus which generally consists of a single membrane-bound compartment in Apicomplexans. From here, proteins are trafficked to various cellular compartments or to the cell surface.
Like other apicomplexans, Plasmodium species have several cellular structures at the apical end of the parasite that serve as specialized organelles for secreting effectors into the host. The most prominent are the bulbous rhoptries which contain parasite proteins involved in invading the host cell and modifying the host once inside. Adjacent to the rhoptries are smaller structures termed micronemes that contain parasite proteins required for motility as well as recognizing and attaching to host cells. Spread throughout the parasite are secretory vesicles called dense granules that contain parasite proteins involved in modifying the membrane that separates the parasite from the host, termed the parasitophorous vacuole.
Species of Plasmodium also contain two large membrane-bound organelles of endosymbiotic origin, the mitochondrion and the apicoplast, both of which play key roles in the parasite's metabolism. Unlike mammalian cells which contain many mitochondria, Plasmodium cells contain a single large mitochondrion that coordinates its division with that of the Plasmodium cell. Like in other eukaryotes, the Plasmodium mitochondrion is capable of generating energy in the form of ATP via the citric acid cycle; however, this function is only required for parasite survival in the insect host, and is not needed for growth in red blood cells. A second organelle, the apicoplast, is derived from a secondary endosymbiosis event, in this case the acquisition of a red alga by the Plasmodium ancestor. The apicoplast is involved in the synthesis of various metabolic precursors, including fatty acids, isoprenoids, iron-sulphur clusters, and components of the heme biosynthesis pathway.
Life cycle

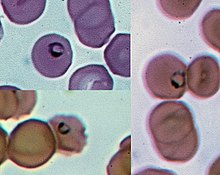
The life cycle of Plasmodium involves several distinct stages in the insect and vertebrate hosts. Parasites are generally introduced into a vertebrate host by the bite of an insect host (generally a mosquito, with the exception of some Plasmodium species of reptiles). Parasites first infect the liver or other tissue, where they undergo a single large round of replication before exiting the host cell to infect erythrocytes. At this point, some species of Plasmodium of primates can form a long-lived dormant stage called a hypnozoite, which can remain in the liver for more than a year. However, for most Plasmodium species, the parasites in infected liver cells are only what are called merozoites. After emerging from the liver, they enter red blood cells, as explained above. They then go through continuous cycles of erythrocyte infection, while a small percentage of parasites differentiate into a sexual stage called a gametocyte which is picked up by an insect host taking a blood meal. In some hosts, invasion of erythrocytes by Plasmodium species can result in disease, called malaria. This can sometimes be severe, rapidly followed by death of the host (e.g. P. falciparum in humans). In other hosts, Plasmodium infection can apparently be asymptomatic.
Even when humans have such subclinical plasmodial infections, there can nevertheless be very large numbers of multiplying parasites concealed in, particularly, the spleen and bone marrow. Certainly, this applies in the case of P. vivax. These hidden parasites (in addition to hypnozoites) are thought to be the origin of instances of recurrent P. vivax malaria.

Within the red blood cells, the merozoites grow first to a ring-shaped form and then to a larger form called a trophozoite. Trophozoites then mature to schizonts which divide several times to produce new merozoites. The infected red blood cell eventually bursts, allowing the new merozoites to travel within the bloodstream to infect new red blood cells. Most merozoites continue this replicative cycle, however some merozoites upon infecting red blood cells differentiate into male or female sexual forms called gametocytes. These gametocytes circulate in the blood until they are taken up when a mosquito feeds on the infected vertebrate host, taking up blood which includes the gametocytes.
In the mosquito, the gametocytes move along with the blood meal to the mosquito's midgut. Here the gametocytes develop into male and female gametes which fertilize each other, forming a zygote. Zygotes then develop into a motile form called an ookinete, which penetrates the wall of the midgut. Upon traversing the midgut wall, the ookinete embeds into the gut's exterior membrane and develops into an oocyst. Oocysts divide many times to produce large numbers of small elongated sporozoites. These sporozoites migrate to the salivary glands of the mosquito where they can be injected into the blood of the next host the mosquito bites, repeating the cycle.
Evolution and taxonomy

Taxonomy
Plasmodium belongs to the phylum Apicomplexa, a taxonomic group of single-celled parasites with characteristic secretory organelles at one end of the cell. Within Apicomplexa, Plasmodium is within the order Haemosporida, a group that includes all apicomplexans that live within blood cells. Based on the presence of the pigment hemozoin and the method of asexual reproduction, the order is further split into four families, of which Plasmodium is in the family Plasmodiidae.
The genus Plasmodium consists of over 200 species, generally described on the basis of their appearance in blood smears of infected vertebrates. These species have been categorized on the basis of their morphology and host range into 14 subgenera:
- Subgenus Asiamoeba (Telford, 1988) – reptiles
- Subgenus Bennettinia (Valkiunas, 1997) – birds
- Subgenus Carinamoeba (Garnham, 1966) – reptiles
- Subgenus Giovannolaia (Corradetti, et al. 1963) – birds
- Subgenus Haemamoeba (Corradetti, et al. 1963) – birds
- Subgenus Huffia (Corradetti, et al. 1963) – birds
- Subgenus Lacertamoeba (Telford, 1988) – reptiles
- Subgenus Laverania (Bray, 1958) – great apes, humans
- Subgenus Novyella (Corradetti, et al. 1963) – birds
- Subgenus Ophidiella (Telford, 1988) – reptiles
- Subgenus Paraplasmodium (Telford, 1988) – reptiles
- Subgenus Plasmodium (Bray, 1955) – monkeys and apes
- Subgenus Sauramoeba (Garnham, 1966) – reptiles
- Subgenus Vinckeia (Garnham, 1964) – mammals inc. primates
Species infecting monkeys and apes with the exceptions of P. falciparum and P. reichenowi (which together make up the subgenus Laverania) are classified in the subgenus Plasmodium. Parasites infecting other mammals including some primates (lemurs and others) are classified in the subgenus Vinckeia. The five subgenera Bennettinia, Giovannolaia, Haemamoeba, Huffia, and Novyella contain the known avian malarial species. The remaining subgenera: Asiamoeba, Carinamoeba, Lacertamoeba, Ophidiella, Paraplasmodium, and Sauramoeba contain the diverse groups of parasites found to infect reptiles.
Phylogeny
More recent studies of Plasmodium species using molecular methods have implied that the group's evolution has not perfectly followed taxonomy. Many Plasmodium species that are morphologically similar or infect the same hosts turn out to be only distantly related. In the 1990s, several studies sought to evaluate evolutionary relationships of Plasmodium species by comparing ribosomal RNA and a surface protein gene from various species, finding the human parasite P. falciparum to be more closely related to avian parasites than to other parasites of primates. However, later studies sampling more Plasmodium species found the parasites of mammals to form a clade along with the genus Hepatocystis, while the parasites of birds or lizards appear to form a separate clade with evolutionary relationships not following the subgenera:
| ||||||||||||||||||||||||||||
Estimates for when different Plasmodium lineages diverged have differed broadly. Estimates for the diversification of the order Haemosporida range from around 16.2 million to 100 million years ago. There has been particular interest in dating the divergence of the human parasite P. falciparum from other Plasmodium lineages due to its medical importance. For this, estimated dates range from 110,000 to 2.5 million years ago.
Distribution
Plasmodium species are distributed globally. All Plasmodium species are parasitic and must pass between a vertebrate host and an insect host to complete their life cycles. Different species of Plasmodium display different host ranges, with some species restricted to a single vertebrate and insect host, while other species can infect several species of vertebrates and/or insects.
Vertebrates

Plasmodium parasites have been described in a broad array of vertebrate hosts including reptiles, birds, and mammals. While many species can infect more than one vertebrate host, they are generally specific to one of these classes (such as birds).
Humans are primarily infected by five species of Plasmodium, with the overwhelming majority of severe disease and death caused by Plasmodium falciparum. Some species that infect humans can also infect other primates, and zoonoses of certain species (e.g. P. knowlesi) from other primates to humans are common. Non-human primates also contain a variety of Plasmodium species that do not generally infect humans. Some of these can cause severe disease in primates, while others can remain in the host for prolonged periods without causing disease. Many other mammals also carry Plasmodium species, such as a variety of rodents, ungulates, and bats. Again, some species of Plasmodium can cause severe disease in some of these hosts, while many appear not to.
Over 150 species of Plasmodium infect a broad variety of birds. In general each species of Plasmodium infects one to a few species of birds. Plasmodium parasites that infect birds tend to persist in a given host for years or for the life time of the host, although in some cases Plasmodium infections can result in severe illness and rapid death. Unlike with Plasmodium species infecting mammals, those infecting birds are distributed across the globe.
Species from several subgenera of Plasmodium infect diverse reptiles. Plasmodium parasites have been described in most lizard families and, like avian parasites, are spread worldwide. Again, parasites can result either in severe disease or be apparently asymptomatic depending on the parasite and the host.
A number of drugs have been developed over the years to control Plasmodium infection in vertebrate hosts, particularly in humans. Quinine was used as a frontline antimalarial from the 17th century until widespread resistance emerged in the early 20th century. Resistance to quinine spurred the development of a broad array of antimalarial medications through the 20th century including chloroquine, proguanil, atovaquone, sulfadoxine/pyrimethamine, mefloquine, and artemisinin. In all cases, parasites resistant to a given drug have emerged within a few decades of the drugs deployment. To combat this, antimalarial drugs are frequently used in combination, with artemisinin combination therapies currently the gold standard for treatment. In general, antimalarial drugs target the life stages of Plasmodium parasites that reside within vertebrate red blood cells, as these are the stages that tend to cause disease. However, drugs targeting other stages of the parasite life cycle are under development in order to prevent infection in travelers and to prevent transmission of sexual stages to insect hosts.
-
 A clinic for treating human malaria in Tanzania
A clinic for treating human malaria in Tanzania
-
 Over 3000 species of lizard, including the Carolina anole (Anolis carolinensis), carry some 90 kinds of malaria.
Over 3000 species of lizard, including the Carolina anole (Anolis carolinensis), carry some 90 kinds of malaria.
Insects

In addition to a vertebrate host, all Plasmodium species also infect a bloodsucking insect host, generally a mosquito (although some reptile-infecting parasites are transmitted by sandflies). Mosquitoes of the genera Culex, Anopheles, Culiseta, Mansonia and Aedes act as insect hosts for various Plasmodium species. The best studied of these are the Anopheles mosquitoes which host the Plasmodium parasites of human malaria, as well as Culex mosquitoes which host the Plasmodium species that cause malaria in birds. Only female mosquitoes are infected with Plasmodium, since only they feed on the blood of vertebrate hosts. Different species affect their insect hosts differently. Sometimes, insects infected with Plasmodium have reduced lifespan and reduced ability to produce offspring. Further, some species of Plasmodium appear to cause insects to prefer to bite infected vertebrate hosts over non-infected hosts.
History
Charles Louis Alphonse Laveran first described parasites in the blood of malaria patients in 1880. He named the parasite Oscillaria malariae. In 1885, zoologists Ettore Marchiafava and Angelo Celli reexamined the parasite and termed it a member of a new genus, Plasmodium, named for the resemblance to the multinucleate cells of slime molds of the same name. The fact that several species may be involved in causing different forms of malaria was first recognized by Camillo Golgi in 1886. Soon thereafter, Giovanni Batista Grassi and Raimondo Filetti named the parasites causing two different types of human malaria Plasmodium vivax and Plasmodium malariae. In 1897, William Welch identified and named Plasmodium falciparum. This was followed by the recognition of the other two species of Plasmodium which infect humans: Plasmodium ovale (1922) and Plasmodium knowlesi (identified in long-tailed macaques in 1931; in humans in 1965). The contribution of insect hosts to the Plasmodium life cycle was described in 1897 by Ronald Ross and in 1899 by Giovanni Batista Grassi, Amico Bignami and Giuseppe Bastianelli.
In 1966, Cyril Garnham proposed separating Plasmodium into nine subgenera based on host specificity and parasite morphology. This included four subgenera that had previously been proposed for bird-infecting Plasmodium species by A. Corradetti in 1963. This scheme was expanded upon by Sam R. Telford in 1988 when he reclassified Plasmodium parasites that infect reptiles, adding five subgenera. In 1997, G. Valkiunas reclassified the bird-infecting Plasmodium species adding a fifth subgenus: Bennettinia.
See also
Notes
- The plural of Plasmodium is not Plasmodia. Instead multiple species of the genus are referred to as "Plasmodium species".
References
- "CDC – Malaria Parasites – About". CDC: Malaria. U.S. Centers for Disease Control and Prevention. Retrieved 28 December 2015.
- ^ Zilversmit, M.; Perkins, S. "Plasmodium". Tree of Life Web Project. Retrieved 1 June 2016.
- Obado, Samson O; Glover, Lucy; Deitsch, Kirk W. (2016). "The nuclear envelope and gene organization in parasitic protozoa: Specializations associated with disease". Molecular and Biochemical Parasitology. 209 (1–2): 104–113. doi:10.1016/j.molbiopara.2016.07.008. PMID 27475118.
- ^ Jimenez-Ruiz, Elena; Morlon-Guyot, Juliette; Daher, Wassim; Meissner, Markus (2016). "Vacuolar protein sorting mechanisms in apicomplexan parasites". Molecular and Biochemical Parasitology. 209 (1–2): 18–25. doi:10.1016/j.molbiopara.2016.01.007. PMC 5154328. PMID 26844642.
- Counihan, Natalie A.; Kalanon, Ming; Coppel, Ross L.; De Koning-Ward, Tania F. (2013). "Plasmodium rhoptry proteins: Why order is important". Trends in Parasitology. 29 (5): 228–36. doi:10.1016/j.pt.2013.03.003. PMID 23570755.
- ^ Kemp, Louise E.; Yamamoto, Masahiro; Soldati-Favre, Dominique (2013). "Subversion of host cellular functions by the apicomplexan parasites". FEMS Microbiology Reviews. 37 (4): 607–31. doi:10.1111/1574-6976.12013. PMID 23186105.
- ^ Sheiner, Lilach; Vaidya, Akhil B.; McFadden, Geoffrey I. (2013). "The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp". Current Opinion in Microbiology. 16 (4): 452–8. doi:10.1016/j.mib.2013.07.003. PMC 3767399. PMID 23927894.
- McFadden, Geoffrey Ian; Yeh, Ellen (2017). "The apicoplast: Now you see it, now you don't". International Journal for Parasitology. 47 (2–3): 137–144. doi:10.1016/j.ijpara.2016.08.005. PMC 5406208. PMID 27773518.
- Dooren, Giel; Striepen, Boris (June 26, 2013). "The Algal Past and Parasite Present of the Apicoplast". Annual Review of Microbiology. 67: 271–289. doi:10.1146/annurev-micro-092412-155741. PMID 23808340.
- ^ Vernick, K.D.; Oduol, F.; Lazarro, B.P.; Glazebrook, J.; Xu, J.; Riehle, M.; Li, J. (2005). "Molecular Genetics of Mosquito Resistance to Malaria Parasites". In Sullivan, D; Krishna, S. (eds.). Malaria: Drugs, Disease, and Post-genomic Biology. Springer. p. 384. ISBN 978-3-540-29088-9.
- ^ "CDC – Malaria Parasites – Biology". CDC: Malaria. U.S. Centers for Disease Control and Prevention. Retrieved 28 December 2015.
- Markus, M. B. (2011). "Malaria: Origin of the Term 'Hypnozoite'". Journal of the History of Biology. 44 (4): 781–786. doi:10.1007/s10739-010-9239-3. PMID 20665090. S2CID 1727294.
- Vaughan, Ashley M.; Kappe, Stefan H. I. (2017). "Malaria Parasite Liver Infection and Exoerythrocytic Biology". Cold Spring Harbor Perspectives in Medicine. 7 (6): a025486. doi:10.1101/cshperspect.a025486. PMC 5453383. PMID 28242785.
- Markus, M. B. (2022). "Theoretical origin of genetically homologous Plasmodium vivax malarial recurrences". Southern African Journal of Infectious Diseases. 37 (1): 369. doi:10.4102/sajid.v37i1.369. PMC 8991251. PMID 35399558.
- Morrison, David A. (2009). "Evolution of the Apicomplexa: Where are we now?". Trends in Parasitology. 25 (8): 375–82. doi:10.1016/j.pt.2009.05.010. PMID 19635681.
- Votypka J. "Haemospororida Danielewski 1885". Tree of Life. Retrieved 1 May 2018.
- ^ Perkins, S. L. (2014). "Malaria's Many Mates: Past, Present, and Future of the Systematics of the Order Haemosporida". Journal of Parasitology. 100 (1): 11–25. doi:10.1645/13-362.1. PMID 24059436. S2CID 21291855.
- ^ Martinsen, E. S.; Perkins, S. L. (2013). "The Diversity of Plasmodium and other Haemosporidians: The Intersection of Taxonomy, Phylogenetics, and Genomics". In Carlton, J.M.; Perkins, S.L.; Deitsch, K.W. (eds.). Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Caister Academic Press. pp. 1–15. ISBN 978-1908230072.
- ^ Valkiunas, Gediminas (2004). "Brief Historical Summary". Avian Malaria Parasites and Other Haemosporidia. CRC Press. pp. 9–15. ISBN 9780415300971.
- ^ Telford S (1988). "A contribution to the systematics of the reptilian malaria parasites, family Plasmodiidae (Apicomplexa: Haemosporina)". Bulletin of the Florida State Museum Biological Sciences. 34 (2): 65–96. Archived from the original on 2018-09-26. Retrieved 2014-03-25.
- Rich, S.; Ayala, F (2003). Progress in Malaria Research: the Case for Phylogenetics. Advances in Parasitology. Vol. 54. pp. 255–80. doi:10.1016/S0065-308X(03)54005-2. ISBN 978-0-12-031754-7. PMID 14711087.
- Martinsen ES, Perkins SL, Schall JJ (April 2008). "A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches". Molecular Phylogenetics and Evolution. 47 (1): 261–273. doi:10.1016/j.ympev.2007.11.012. PMID 18248741.
- Tatem AJ; Jia P; Ordanovich D; Falkner M; Huang Z; Howes R; Hay S; Gething, P W; Smith, D L; et al. (2017). "The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics". Lancet Infect Dis. 17 (1): 98–107. doi:10.1016/S1473-3099(16)30326-7. PMC 5392593. PMID 27777030.
- ^ Manguin, S.; Carnevale, P.; Mouchet, J.; Coosemans, M.; Julvez, J.; Richard-Lenoble, D.; Sircoulon, J. (2008). Biodiversity of Malaria in the world. John Libbey. pp. 13–15. ISBN 978-2-7420-0616-8. Retrieved 15 March 2018.
- ^ Scully, Erik J.; Kanjee, Usheer; Duraisingh, Manoj T. (2017). "Molecular interactions governing host-specificity of blood stage malaria parasites". Current Opinion in Microbiology. 40: 21–31. doi:10.1016/j.mib.2017.10.006. PMC 5733638. PMID 29096194.
- Nunn, C.; Altizer, S. (2006). Infectious Diseases in Primates: Behavior, Ecology and Evolution (1st ed.). Oxford University Press. pp. 253–254. ISBN 978-0198565840. Retrieved 16 March 2018.
- Templeton TJ, Martinsen E, Kaewthamasorn M, Kaneko O (2016). "The rediscovery of malaria parasites of ungulates". Parasitology. 143 (12): 1501–1508. doi:10.1017/S0031182016001141. PMID 27444556. S2CID 22397021.
- ^ Valkiunas, Gediminas (2004). "Specificity and general Principles of Species Identification". Avian Malaria Parasites and Other Haemosporidia. CRC Press. pp. 67–81. ISBN 9780415300971.
- Valkiunas, Gediminas (2004). "General Section - Life Cycle and Morphology of Plasmodiidae Species". Avian Malaria Parasites and Other Haemosporidia. CRC Press. pp. 27–35. ISBN 9780415300971.
- Valkiunas, Gediminas (2004). "Pathogenicity". Avian Malaria Parasites and Other Haemosporidia. CRC Press. pp. 83–111. ISBN 9780415300971.
- ^ Zug, G. R.; Vitt, L. J., eds. (2012). Herpetology: An Introductory Biology of Amphibians and Reptiles. Academic Press. p. 152. ISBN 978-0127826202. Retrieved 16 March 2018.
- ^ Blasco, Benjamin; Leroy, Didier; Fidock, David A. (2017). "Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic". Nature Medicine. 23 (8): 917–928. doi:10.1038/nm.4381. PMC 5747363. PMID 28777791.
- Cowman, Alan F; Healer, Julie; Marapana, Danushka; Marsh, Kevin (2016). "Malaria: Biology and Disease". Cell. 167 (3): 610–624. doi:10.1016/j.cell.2016.07.055. PMID 27768886.
- Haldar, Kasturi; Bhattacharjee, Souvik; Safeukui, Innocent (2018). "Drug resistance in Plasmodium". Nature Reviews Microbiology. 16 (3): 156–170. doi:10.1038/nrmicro.2017.161. PMC 6371404. PMID 29355852.
- Poonam; Gupta, Yash; Gupta, Nikesh; Singh, Snigdha; Wu, Lidong; Chhikara, Bhupender Singh; Rawat, Manmeet; Rathi, Brijesh (2018). "Multistage inhibitors of the malaria parasite: Emerging hope for chemoprotection and malaria eradication". Medicinal Research Reviews. 38 (5): 1511–1535. doi:10.1002/med.21486. PMID 29372568. S2CID 25711437.
- Crompton, Peter D.; Moebius, Jacqueline; Portugal, Silvia; Waisberg, Michael; Hart, Geoffrey; Garver, Lindsey S.; Miller, Louis H.; Barillas-Mury, Carolina; Pierce, Susan K. (2014). "Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease". Annual Review of Immunology. 32 (1): 157–187. doi:10.1146/annurev-immunol-032713-120220. PMC 4075043. PMID 24655294.
- ^ Busula, Annette O.; Verhulst, Niels O.; Bousema, Teun; Takken, Willem; De Boer, Jetske G. (2017). "Mechanisms of Plasmodium -Enhanced Attraction of Mosquito Vectors". Trends in Parasitology. 33 (12): 961–973. doi:10.1016/j.pt.2017.08.010. PMID 28942108.
- Stanczyk, Nina M.; Mescher, Mark C.; De Moraes, Consuelo M. (2017). "Effects of malaria infection on mosquito olfaction and behavior: Extrapolating data to the field". Current Opinion in Insect Science. 20: 7–12. doi:10.1016/j.cois.2017.02.002. PMID 28602239.
- Mitchell, Sara N.; Catteruccia, Flaminia (2017). "Anopheline Reproductive Biology: Impacts on Vectorial Capacity and Potential Avenues for Malaria Control". Cold Spring Harbor Perspectives in Medicine. 7 (12): a025593. doi:10.1101/cshperspect.a025593. PMC 5710097. PMID 28389513.
- ^ "The History of Malaria, an Ancient Disease". U.S. Centers for Disease Control and Prevention. Retrieved 31 May 2016.
- ^ McFadden, G. I. (2012). "Plasmodia – don't". Trends Parasitol. 28 (8): 306. doi:10.1016/j.pt.2012.05.006. PMID 22738856.
- Corradetti A.; Garnham P.C.C.; Laird M. (1963). "New classification of the avian malaria parasites". Parassitologia. 5: 1–4.
- Valkiunas, G. (1997). "Bird Haemosporidia". Acta Zoologica Lituanica. 3–5: 1–607. ISSN 1392-1657.
Further reading
Identification
- Garnham, P. C. (1966). Malaria Parasites And Other Haemosporidia. Oxford: Blackwell. ISBN 978-0397601325.
- Valkiunas, Gediminas (2005). Avian Malaria Parasites and Other Haemosporidia. Boca Raton: CRC Press. ISBN 9780415300971.
Biology
- Baldacci, P.; Ménard, R. (October 2004). "The elusive malaria sporozoite in the mammalian host". Mol. Microbiol. 54 (2): 298–306. doi:10.1111/j.1365-2958.2004.04275.x. PMID 15469504. S2CID 30488807.
- Bledsoe, G. H. (December 2005). "Malaria primer for clinicians in the United States" (PDF). South. Med. J. 98 (12): 1197–204, quiz 1205, 1230. doi:10.1097/01.smj.0000189904.50838.eb. PMID 16440920. S2CID 30660702. Archived from the original (PDF) on 2009-03-26.
- Shortt, H. E. (1951). "Life-cycle of the mammalian malaria parasite". Br. Med. Bull. 8 (1): 7–9. doi:10.1093/oxfordjournals.bmb.a074057. PMID 14944807.
History
- Slater, L. B. (2005). "Malarial birds: modeling infectious human disease in animals". Bull Hist Med. 79 (2): 261–94. doi:10.1353/bhm.2005.0092. PMID 15965289. S2CID 23594155.
External links
| Taxon identifiers | |
|---|---|
| Plasmodium | |