| Revision as of 14:37, 20 March 2024 editPointlessUsername (talk | contribs)Extended confirmed users6,293 editsNo edit summaryTag: Visual edit← Previous edit | Latest revision as of 22:12, 3 September 2024 edit undoBD2412 (talk | contribs)Autopatrolled, IP block exemptions, Administrators2,453,987 editsm →top: Clean up spacing around commas and other punctuation fixes, replaced: ,S → , STag: AWB | ||
| Line 48: | Line 48: | ||
| | Section6 = | | Section6 = | ||
| }} | }} | ||
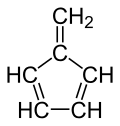
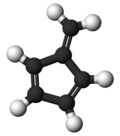
| '''Fulvene''' (pentafulvene) is a ] with the formula (CH=CH)<sub>2</sub>C=CH<sub>2</sub>. It is a prototype of a ] hydrocarbon.<ref>{{cite journal|author1=Preethanuj Preethalayam |author2=Syam krishnan, K.|author3=Sreeja Thulasi |author4= Sarath Chand,S. |author5=Jomy Joseph | '''Fulvene''' (pentafulvene) is a ] with the formula (CH=CH)<sub>2</sub>C=CH<sub>2</sub>. It is a prototype of a ] hydrocarbon.<ref>{{cite journal|author1=Preethanuj Preethalayam |author2=Syam krishnan, K.|author3=Sreeja Thulasi |author4= Sarath Chand, S. |author5=Jomy Joseph | ||
| |author6= Vijay Nair | |author6= Vijay Nair | ||
| |author7=Florian Jaroschik|author8=K.V.Radhakrishnan | |author7=Florian Jaroschik|author8=K.V.Radhakrishnan | ||
| Line 63: | Line 63: | ||
| | doi = 10.1021/cr60251a002}}</ref> but substituted derivatives (]) are numerous. They are mainly of interest as ]s and precursors to ligands in ]. | | doi = 10.1021/cr60251a002}}</ref> but substituted derivatives (]) are numerous. They are mainly of interest as ]s and precursors to ligands in ]. | ||
| Fulvene is an isomer of ], which when irradiated at 237 to 254 |
Fulvene is an isomer of ], which when irradiated at 237 to 254 nm forms small amounts of fulvene along with ].<ref>{{Cite journal |last=Kaplan |first=Louis |last2=Wilzbach |first2=K. E. |date=1968 |title=Photolysis of benzene vapor. Benzvalene formation at wavelengths 2537-2370 A |url=https://pubs.acs.org/doi/abs/10.1021/ja01014a086 |journal=Journal of the American Chemical Society |language=en |volume=90 |issue=12 |pages=3291–3292 |doi=10.1021/ja01014a086 |issn=0002-7863}}</ref> | ||
| == See also == | == See also == | ||
Latest revision as of 22:12, 3 September 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 5-Methylidenecyclopenta-1,3-diene | |||
| Other names
Fulvene 5-Methylene-1,3-cyclopentadiene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H6 | ||
| Molar mass | 78.114 g·mol | ||
| Magnetic susceptibility (χ) | -42.9·10 cm/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Fulvene (pentafulvene) is a hydrocarbon with the formula (CH=CH)2C=CH2. It is a prototype of a cross-conjugated hydrocarbon. Fulvene is rarely encountered, but substituted derivatives (fulvenes) are numerous. They are mainly of interest as ligands and precursors to ligands in organometallic chemistry.
Fulvene is an isomer of benzene, which when irradiated at 237 to 254 nm forms small amounts of fulvene along with benzvalene.
See also
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 379. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Preethanuj Preethalayam; Syam krishnan, K.; Sreeja Thulasi; Sarath Chand, S.; Jomy Joseph; Vijay Nair; Florian Jaroschik; K.V.Radhakrishnan (2017). "Recent Advances in the Chemistry of Pentafulvenes". Chemical Reviews. 117 (5): 3930–3989. doi:10.1021/acs.chemrev.6b00210. PMID 28151643.
- Bergmann, E. D. (1968). "Fulvenes and Substituted Fulvenes". Chemical Reviews. 68: 41–84. doi:10.1021/cr60251a002.
- Kaplan, Louis; Wilzbach, K. E. (1968). "Photolysis of benzene vapor. Benzvalene formation at wavelengths 2537-2370 A". Journal of the American Chemical Society. 90 (12): 3291–3292. doi:10.1021/ja01014a086. ISSN 0002-7863.