| Revision as of 09:46, 2 December 2010 editNono64 (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers96,246 editsm Category:Phenylpropanoids← Previous edit | Revision as of 11:49, 12 March 2011 edit undoNono64 (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers96,246 editsm natural phenol-stubNext edit → | ||
| Line 38: | Line 38: | ||
| {{Neurotransmitter metabolism intermediates}} | {{Neurotransmitter metabolism intermediates}} | ||
| ] | |||
| ] | ] | ||
| {{ |
{{natural phenol-stub}} | ||
Revision as of 11:49, 12 March 2011
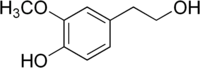
| |
| Names | |
|---|---|
| IUPAC name 4-(2-Hydroxyethyl)-2-methoxyphenol | |
| Other names Homovanillic alcohol; MOPET; 3-Methoxy-4-hydroxyphenylethanol; 3-Methoxy-4-hydroxyphenethyl alcohol; 4-Hydroxy-3-methoxyphenethanol, 4-Hydroxy-3-methoxyphenethyl alcohol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.017.433 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C9H12O3 |
| Molar mass | 168.19 g/mol |
| Melting point | 40-42 °C |
| Hazards | |
| Flash point | 113 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Homovanillyl alcohol is a metabolite of hydroxytyrosol, which in turn is a metabolite of the neurotransmitter dopamine.
See also
References
| Neurotransmitter metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catecholamines |
| ||||||||||
| Tryptophan→Serotonin |
| ||||||||||
| Serotonin→Melatonin | |||||||||||
| Trace amines | |||||||||||
| GABA | |||||||||||