| Revision as of 11:14, 9 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit | Revision as of 01:09, 15 December 2011 edit undo70.176.165.122 (talk) →Production of the disulfite ionTag: possible vandalismNext edit → | ||
| Line 31: | Line 31: | ||
| ==Chemistry== | ==Chemistry== | ||
| balls tee hee | |||
| ===Production of the disulfite ion=== | |||
| The disulfite ion is a ] of the ] ion (HSO<sub>3</sub><sup>−</sup>). It can arise from: | |||
| ''']''' | |||
| In aqueous solution, the disulfite ion is formed in minor amounts by dehydration of bisulfite in an equilibrium: | |||
| : 2 HSO<sub>3</sub><sup>−</sup> (aq) ] S<sub>2</sub>O<sub>5</sub><sup>2−</sup> (aq) + H<sub>2</sub>O (l) | |||
| Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.<ref name=Bassam>Bassam Z. Shakhashiri: The University of Wisconsin Press @Google Books, 1992, p.9</ref> | |||
| In fact, disulfite is the ion of ] (pyrosulfurous acid), which originates from ] in accordance with the dehydration reaction above:<br /> | |||
| :2 H<sub>2</sub>SO<sub>3</sub> → 2 HSO<sub>3</sub><sup>−</sup> + 2 H<sup>+</sup> → H<sub>2</sub>S<sub>2</sub>O<sub>5</sub> + H<sub>2</sub>O | |||
| '''addition''' | |||
| The disulfite ion also arises from the addition of ] to the ] ion:<br /> | |||
| {| class="wikitable" | |||
| |- | |||
| |HSO<sub>3</sub><sup>−</sup> ] SO<sub>3</sub><sup>2−</sup> + H<sup>+</sup><br /><br />SO<sub>3</sub><sup>2−</sup> + SO<sub>2</sub> ] S<sub>2</sub>O<sub>5</sub><sup>2−</sup> || || ] | |||
| |} | |||
| ===Other reactions=== | ===Other reactions=== | ||
Revision as of 01:09, 15 December 2011
Not to be confused with Bisulfite.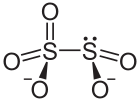
| |
| Names | |
|---|---|
| IUPAC name disulfite | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | S2O5 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
A disulfite, commonly known as metabisulfite, is a chemical compound containing the disulfite ion (metabisulfite ion) .
Chemistry
balls tee hee
Other reactions
In aqueous solution, disulfite salts decompose with acids:
S2O5 + H → HSO3 + SO2
Examples of disulfites
- sodium metabisulfite (E223) and potassium metabisulfite (E224) are used as a preservative and antioxidant in food.
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.