| Revision as of 19:17, 9 August 2011 editStemonitis (talk | contribs)Extended confirmed users179,488 edits citation no longer in press: add page nos.; etc.← Previous edit | Revision as of 13:59, 4 October 2011 edit undoCitation bot 1 (talk | contribs)Bots130,044 editsm Add: last2, first2, last3, first3, last4, first4, last5, first5, last6, first6, last7, first7, last8, first8, last9, first9. Tweak: title, last2, first2, last3, first3, last4, first4, last5, first5, last6, first6, last7, first7, last8, first8,Next edit → | ||
| Line 38: | Line 38: | ||
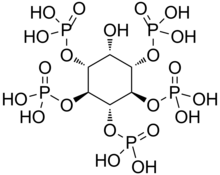
| '''Inositol pentakisphosphate''' (abbreviated '''IP5''') is a molecule derived from ] by adding a ] with the help of ] (IPMK). It is believed to be one of the many ]s in the ] family. It "is implicated in a wide array of biological and pathophysiological responses, including tumorigenesis, invasion and metastasis, therefore specific inhibitors of the kinase may prove useful in cancer therapy."<ref name= "IP Promotes apoptosis">{{cite journal | pmid=14755253 | year=2004 | last1=Piccolo | first1=E. | last2=Vignati | first2=S. | last3=Maffucci | first3=T. | last4=Innominato | first4=P. F. | last5=Riley | first5=A. M. | last6=Potter | first6=B. V. | last7=Pandolfi | first7=P. P. | last8=Broggini | first8=M. | last9=Iacobelli | first9=S. | title=Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway | volume=23 | issue=9 | pages=1754–1765 | doi=10.1038/sj.onc.1207296 | journal=]}}</ref> | '''Inositol pentakisphosphate''' (abbreviated '''IP5''') is a molecule derived from ] by adding a ] with the help of ] (IPMK). It is believed to be one of the many ]s in the ] family. It "is implicated in a wide array of biological and pathophysiological responses, including tumorigenesis, invasion and metastasis, therefore specific inhibitors of the kinase may prove useful in cancer therapy."<ref name= "IP Promotes apoptosis">{{cite journal | pmid=14755253 | year=2004 | last1=Piccolo | first1=E. | last2=Vignati | first2=S. | last3=Maffucci | first3=T. | last4=Innominato | first4=P. F. | last5=Riley | first5=A. M. | last6=Potter | first6=B. V. | last7=Pandolfi | first7=P. P. | last8=Broggini | first8=M. | last9=Iacobelli | first9=S. | title=Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway | volume=23 | issue=9 | pages=1754–1765 | doi=10.1038/sj.onc.1207296 | journal=]}}</ref> | ||
| IP5 also plays a role in defense signaling in plants. It potentiates the interaction of the plant hormone ] by its receptor.<ref>{{cite journal |last=Sheard |first=L. B. |year=2010 |title=Jasmonate perception by inositol |
IP5 also plays a role in defense signaling in plants. It potentiates the interaction of the plant hormone ] by its receptor.<ref>{{cite journal |last=Sheard |first=L. B. |year=2010 |title=Jasmonate perception by inositol phosphate-potentiated COI1-JAZ co-receptor|journal=] |volume=468 |pages=400–405 |doi=10.1038/nature09430 |pmid=20927106 |pmc=2988090 |issue=7322 |last2=Tan |first2=Xu |last3=Mao |first3=Haibin |last4=Withers |first4=John |last5=Ben-Nissan |first5=Gili |last6=Hinds |first6=Thomas R. |last7=Kobayashi |first7=Yuichi |last8=Hsu |first8=Fong-Fu |last9=Sharon |first9=Michal}}</ref><ref>{{cite journal |last=Mosblech |first=A. |year=2010 |title=Jasmonic acid perception by COI1 involves inositol polyphosphates in ''Arabidopsis thaliana'' |journal=] | volume=65 |issue=6 |pages=949–957 |doi=10.1111/j.1365-313X.2011.04480.x |pmid=21205029 |last2=Thurow |first2=Corinna |last3=Gatz |first3=Christiane |last4=Feussner |first4=Ivo |last5=Heilmann |first5=Ingo}}</ref> | ||
| ==References== | ==References== | ||
Revision as of 13:59, 4 October 2011
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H17O21P5 |
| Molar mass | 580.050 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Inositol pentakisphosphate (abbreviated IP5) is a molecule derived from inositol tetrakisphosphate by adding a phosphate group with the help of inositol polyphosphate multikinase (IPMK). It is believed to be one of the many second messengers in the inositol phosphate family. It "is implicated in a wide array of biological and pathophysiological responses, including tumorigenesis, invasion and metastasis, therefore specific inhibitors of the kinase may prove useful in cancer therapy."
IP5 also plays a role in defense signaling in plants. It potentiates the interaction of the plant hormone JA-Ile by its receptor.
References
- Sigma Aldrich
- Piccolo, E.; Vignati, S.; Maffucci, T.; Innominato, P. F.; Riley, A. M.; Potter, B. V.; Pandolfi, P. P.; Broggini, M.; Iacobelli, S. (2004). "Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway". Oncogene. 23 (9): 1754–1765. doi:10.1038/sj.onc.1207296. PMID 14755253.
- Sheard, L. B.; Tan, Xu; Mao, Haibin; Withers, John; Ben-Nissan, Gili; Hinds, Thomas R.; Kobayashi, Yuichi; Hsu, Fong-Fu; Sharon, Michal (2010). "Jasmonate perception by inositol phosphate-potentiated COI1-JAZ co-receptor". Nature. 468 (7322): 400–405. doi:10.1038/nature09430. PMC 2988090. PMID 20927106.
- Mosblech, A.; Thurow, Corinna; Gatz, Christiane; Feussner, Ivo; Heilmann, Ingo (2010). "Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana". The Plant Journal. 65 (6): 949–957. doi:10.1111/j.1365-313X.2011.04480.x. PMID 21205029.
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |