| Revision as of 07:16, 1 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to watched fields - updated 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Revision as of 22:13, 14 January 2012 edit undoZéroBot (talk | contribs)704,777 editsm r2.7.1) (Robot: Adding hu:OxolaminNext edit → | ||
| Line 66: | Line 66: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Revision as of 22:13, 14 January 2012
Pharmaceutical compound | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.267 |
| Chemical and physical data | |
| Formula | C14H19N3O |
| Molar mass | 245.32 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Oxolamine is a cough suppressant.
Synthesis
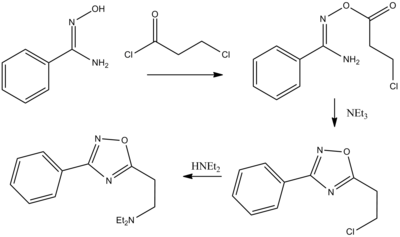
The syntheses of 1,2,4-oxadiazole systems all rely on hydroxylamine for providing a preformed N-O linkage. The preparation of the respiratory anti-inflammatory and antitussive agent oxolamine starts by acylation of the alkoxide from the N-hydroxyamidine with 3-chloropropionyl chloride; the presence of the negative charge on oxygen results in the formation of the O-acylated product. Treatment of that intermediate with triethylamine leads to cyclization via imine formation to afford the 1,2,4-oxadiazole. Displacement of the terminal chlorine with diethylamine gives the corresponding amine oxolamine.
Categories: