| Revision as of 18:04, 10 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validation← Previous edit | Revision as of 23:04, 11 April 2012 edit undoAvocatoBot (talk | contribs)76,551 editsm r2.7.1) (Robot: Adding fa:اوروبولNext edit → | ||
| Line 34: | Line 34: | ||
| ] | ] | ||
| ] | |||
Revision as of 23:04, 11 April 2012
| |
| Names | |
|---|---|
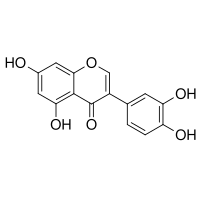
| IUPAC name 3-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | |
| Other names
Isoluteolin Santol 5,7,3',4'-Tetrahydroxyisoflavone 3',4',5,7-Tetrahydroxyisoflavone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 292790 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C15H10O6 |
| Molar mass | 286.23 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Orobol is one of several known isoflavones. It can be isolated from Aspergillus niger or Streptomyces neyagawaensis. It is a potent inhibitor of phosphatidylinositol kinase.
References
- Orobol on curehunter.com
- Isoflavonoids, genistein, psi-tectorigenin, and orobol, increase cytoplasmic free calcium in isolated rat hepatocytes. Tomonaga, T : Mine, T : Kojima, I : Taira, M : Hayashi, H : Isono, K, 1992
| Isoflavones and their glycosides | |
|---|---|
| Isoflavones | |
| O-methylated isoflavones | |
| Glycosides | |
| Prenylated isoflavones | |
| Pyranoisoflavones | |
| Derivatives | |
| Synthetic | |