| Revision as of 18:10, 21 November 2011 editChris Capoccia (talk | contribs)Extended confirmed users86,710 editsNo edit summary← Previous edit | Revision as of 18:10, 21 November 2011 edit undoCitation bot (talk | contribs)Bots5,461,337 editsm Add: year, last1, first1, last2, first2, last3, first3, title, volume, pages, journal, last4, first4, issue, last5, first5, last6, first6, last7, first7, last8, first8, last9, first9. Formatted dashes. | [[User:Chris Capoccia|C...Next edit → | ||
| Line 40: | Line 40: | ||
| '''Coptisine''' is an ] found in Chinese goldthread ('']'').<ref>{{MEDRS}}</ref> Famous for the bitter taste that it produces, it is used in ] along with the related compound ] for treating digestive disorders caused by bacterial infections.{{fact}} | '''Coptisine''' is an ] found in Chinese goldthread ('']'').<ref>{{MEDRS}}</ref> Famous for the bitter taste that it produces, it is used in ] along with the related compound ] for treating digestive disorders caused by bacterial infections.{{fact}} | ||
| Also found in ] and has also been detected in ].<ref>{{cite journal |pmid=13710637}}</ref> | Also found in ] and has also been detected in ].<ref>{{cite journal |pmid=13710637 |year=1961 |last1=Hakim |first1=SA |last2=Mijovic |first2=V |last3=Walker |first3=J |title=Distribution of certain poppy-fumaria alkaloids and a possible link with the incidence of glaucoma |volume=189 |pages=198–201 |journal=Nature}}</ref> | ||
| Coptisine has been found to reversibly inhibit ] in mice, pointing to a potential role as a natural antidepressant.<ref>{{cite journal |pmid=11833714}}</ref> However, this may also imply a hazard for those taking other medications or with a natural functional disorder in Monoamine oxidase A.{{fact}} | Coptisine has been found to reversibly inhibit ] in mice, pointing to a potential role as a natural antidepressant.<ref>{{cite journal |pmid=11833714 |year=2001 |last1=Ro |first1=JS |last2=Lee |first2=SS |last3=Lee |first3=KS |last4=Lee |first4=MK |title=Inhibition of type a monoamine oxidase by coptisine in mouse brain |volume=70 |issue=6 |pages=639–45 |journal=Life sciences}}</ref> However, this may also imply a hazard for those taking other medications or with a natural functional disorder in Monoamine oxidase A.{{fact}} | ||
| Coptisine was found to be toxic to larval ] and a variety of human cell lines, potentially implying a therapeutic effect on cancer or alternatively a generally toxic character. The same authors illustrate a four-step process to produce Coptisine from ].<ref>{{cite journal |pmid=11482767}}</ref> | Coptisine was found to be toxic to larval ] and a variety of human cell lines, potentially implying a therapeutic effect on cancer or alternatively a generally toxic character. The same authors illustrate a four-step process to produce Coptisine from ].<ref>{{cite journal |pmid=11482767 |year=2001 |last1=Colombo |first1=ML |last2=Bugatti |first2=C |last3=Mossa |first3=A |last4=Pescalli |first4=N |last5=Piazzoni |first5=L |last6=Pezzoni |first6=G |last7=Menta |first7=E |last8=Spinelli |first8=S |last9=Johnson |first9=F |title=Cytotoxicity evaluation of natural coptisine and synthesis of coptisine from berberine |volume=56 |issue=5–7 |pages=403–9 |journal=Farmaco (Societa chimica italiana : 1989)}}</ref> | ||
| ==Footnotes== | ==Footnotes== | ||
Revision as of 18:10, 21 November 2011
 | |
| Names | |
|---|---|
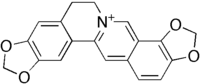
| IUPAC name 6,7-Dihydro-bis(1,3)benzodioxolo (5,6-a:4',5'-g)quinolizinium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H14NO4+ |
| Molar mass | 320.319 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Coptisine is an alkaloid found in Chinese goldthread (Coptis chinensis). Famous for the bitter taste that it produces, it is used in Chinese herbal medicine along with the related compound berberine for treating digestive disorders caused by bacterial infections.
Also found in Greater Celandine and has also been detected in Opium.
Coptisine has been found to reversibly inhibit Monoamine oxidase A in mice, pointing to a potential role as a natural antidepressant. However, this may also imply a hazard for those taking other medications or with a natural functional disorder in Monoamine oxidase A.
Coptisine was found to be toxic to larval brine shrimp and a variety of human cell lines, potentially implying a therapeutic effect on cancer or alternatively a generally toxic character. The same authors illustrate a four-step process to produce Coptisine from Berberine.
Footnotes
- Complementary and Alternative Healing University (Chinese Medicine)
- Hakim, SA; Mijovic, V; Walker, J (1961). "Distribution of certain poppy-fumaria alkaloids and a possible link with the incidence of glaucoma". Nature. 189: 198–201. PMID 13710637.
- Ro, JS; Lee, SS; Lee, KS; Lee, MK (2001). "Inhibition of type a monoamine oxidase by coptisine in mouse brain". Life sciences. 70 (6): 639–45. PMID 11833714.
- Colombo, ML; Bugatti, C; Mossa, A; Pescalli, N; Piazzoni, L; Pezzoni, G; Menta, E; Spinelli, S; Johnson, F (2001). "Cytotoxicity evaluation of natural coptisine and synthesis of coptisine from berberine". Farmaco (Societa chimica italiana : 1989). 56 (5–7): 403–9. PMID 11482767.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |