| Revision as of 15:56, 6 December 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 444112139 of page Sodium_selenite for the Chem/Drugbox validation project (updated: 'ChEMBL').← Previous edit | Revision as of 15:57, 6 December 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 460190558 of page Sodium_silicate for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{Chembox | ||
| | verifiedrevid = |
| verifiedrevid = 447571464 | ||
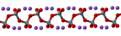
| | ImageFileL1 = Sodium-metasilicate-repeating-unit-2D.png | |||
| | ImageFile = | |||
| | ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | ImageSize = | |||
| | ImageSizeL1 = 121 | |||
| | IUPACName = | |||
| | ImageNameL1 = Structural formula of polymeric sodium silicate | |||
| ⚫ | | OtherNames = | ||
| | ImageFileR1 = Sodium-metasilicate-chain-from-xtal-3D-balls.png | |||
| | ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| | ImageSizeR1 = 121 | |||
| | ImageNameR1 = Ball and stick model of polymeric sodium silicate | |||
| | ImageFile2 = Křemičitan sodný.PNG | |||
| | ImageFile2_Ref = {{chemboximage|correct|??}} | |||
| | ImageSize2 = 244 | |||
| | ImageName2 = Sample of sodium silicate in a vial | |||
| | IUPACName = Sodium metasilicate | |||
| ⚫ | | OtherNames = Liquid glass<br /> | ||
| Waterglass | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | Abbreviations = E550 | |||
| ⚫ | | |
||
| ⚫ | | CASNo = 6834-92-0 | ||
| ⚫ | | ChemSpiderID = |
||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| PubChem = 23266 | ||
| | PubChem_Ref = {{Pubchemcite|correct|pubchem}} | |||
| ⚫ | | InChI = 1/2Na. |
||
| ⚫ | | ChemSpiderID = 21758 | ||
| | InChIKey = BVTBRVFYZUCAKH-NUQVWONBAC | |||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
| EINECS = 229-912-9 | ||
| ⚫ | | UNNumber = 3253 | ||
| ⚫ | | SMILES = ..()=O | ||
| | MeSHName = Sodium+metasilicate | |||
| ⚫ | | |
||
| ⚫ | | RTECS = VV9275000 | ||
| ⚫ | | |
||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 60720 | |||
| ⚫ | | StdInChIKey = |
||
| ⚫ | | SMILES = ..()=O | ||
| ⚫ | | CASNo = |
||
| ⚫ | | StdInChI=1S/2Na.O3Si/c;;1-4(2)3/q2*+1;-2 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| ⚫ | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | ChEMBL = 112302 | |||
| ⚫ | | InChI = 1/2Na.O3Si/c;;1-4(2)3/q2*+1;-2 | ||
| | PubChem = 24934 | |||
| ⚫ | | StdInChIKey = NTHWMYGWWRZVTN-UHFFFAOYSA-N | ||
| | EINECS = 233-267-9 | |||
| ⚫ | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| ⚫ | | RTECS = |
||
| | InChIKey = NTHWMYGWWRZVTN-UHFFFAOYAB | |||
| ⚫ | | UNNumber = |
||
| }} | |||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Na = 2 |
| Na = 2 | ||
| | O = 3 | |||
| | Si = 1 | |||
| | ExactMass = 121.941209749 g mol<sup>-1</sup> | |||
| | Appearance = |
| Appearance = White, opaque crystals | ||
| | Density = | |||
| | Density = 2.4 g cm<sup>-3</sup> | |||
| | MeltingPt = 320 °C (decomp.) | |||
| | MeltingPtC = 1088 | |||
| | BoilingPt = | |||
| | RefractIndex = 1.52 | |||
| | Solubility = 850 g/L (20 °C) | |||
| }} | |||
| | |
| Section3 = {{Chembox Thermochemistry | ||
| | DeltaHf = −1561.43 kJ mol<sup>-1</sup> | |||
| | ExternalMSDS = | |||
| | Entropy = 113.71 J K<sup>−1</sup> mol<sup>−1</sup> | |||
| ⚫ | | EUIndex = |
||
| ⚫ | }} | ||
| | EUClass = Very toxic ('''T+''')<br/>Dangerous for the environment ('''N''') | |||
| | Section4 = {{Chembox Hazards | |||
| ⚫ | | |
||
| | ExternalMSDS = | |||
| | SPhrases = {{S1/2}}, {{S28}}, {{S36/37}}, {{S45}}, {{S61}} | |||
| ⚫ | | EUIndex = 014-010-00-8 | ||
| ⚫ | | NFPA- |
||
| | EUClass = {{Hazchem C}} | |||
| | NFPA-F = 0 | |||
| | NFPA- |
| NFPA-H = 2 | ||
| | |
| NFPA-F = 0 | ||
| ⚫ | | NFPA-R = 0 | ||
| ⚫ | |||
| | RPhrases = {{R34}}, {{R37}} | |||
| ⚫ | | SPhrases = {{S1/2}}, {{S13}}, {{S24/25}}, {{S36/37/39}}, {{S45}} | ||
| ⚫ | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | OtherAnions = ] | |||
| | OtherCpds = ] <br> ] <br> ] | |||
| | OtherCations = ] | |||
| ⚫ | |||
| }} | |||
| }} | }} | ||
Revision as of 15:57, 6 December 2011
| This page contains a copy of the infobox ({{chembox}}) taken from revid 460190558 of page Sodium_silicate with values updated to verified values. |
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium metasilicate | |||
| Other names
Liquid glass Waterglass | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Abbreviations | E550 | ||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | Sodium+metasilicate | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 3253 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | Na2O3Si | ||
| Molar mass | 122.062 g·mol | ||
| Appearance | White, opaque crystals | ||
| Density | 2.4 g cm | ||
| Melting point | 1,088 °C (1,990 °F; 1,361 K) | ||
| Refractive index (nD) | 1.52 | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
113.71 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−1561.43 kJ mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Other anions | Sodium carbonate | ||
| Other cations | Potassium silicate | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound