| Revision as of 07:06, 27 December 2011 editPetergans (talk | contribs)Extended confirmed users13,256 editsNo edit summary← Previous edit | Revision as of 09:11, 15 April 2012 edit undoRezabot (talk | contribs)Extended confirmed users48,194 editsm r2.7.1) (Robot: Adding fa:تفلیک اسیدNext edit → | ||
| Line 60: | Line 60: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Revision as of 09:11, 15 April 2012
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Pentafluoroorthotelluric acid | |||
| Other names Teflic acid | |||
| Identifiers | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.161.534 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | HF5OTe | ||
| Molar mass | 239.6 | ||
| Appearance | colorless solid | ||
| Melting point | 39.1 °C | ||
| Boiling point | 59.7 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | corrosive, toxic | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
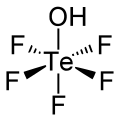
Teflic acid is the chemical compound with the formula HOTeF5. This strong acid is related to orthotelluric acid, Te(OH)6. Teflic acid has octahedral geometry and, Ignoring its bent Te-O-H bond, has point group symmetry C4v.
Preparation
Teflic acid can be prepared from barium tellurate and fluorosulfonic acid:
- 5HOSO2F + BaO2Te(OH)4 → HOTeF5 + 4 H2SO4 + BaSO4
It is also the first hydrolysis product of tellurium hexafluoride:
- TeF6 + H2O → HOTeF5 + HF
Teflates
The conjugate base of teflic acid is called the teflate anion, F5TeO (not to be confused with triflate). Many teflates are known, examples being B(OTeF5)3 and the acid anhydride O(TeF5)2. Pyrolysis of the boron compound gives the dimer (TeF4O)2
- 2 B(OTeF5)3 → 2 B(OTeF5)2F + (OTeF4)2
The teflate anion is known to resist oxidaton. This property has allowed the preparation several highly unusual species such as the hexateflates M(OTeF5)6 (M = As, Sb, Bi). Xenon forms the cation Xe(OTeF5),
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Mercier, H. P.A.; Sanders, J. C. P.; Schrobilgen, G. J. "The Hexakis(pentafluorooxotellurato)pnictate(V) Anions, M(OTeF5) (M = As, Sb, Bi): A Series of Very Weakly Coordinating Anions" Journal of the American Chemical Society, volume 116, 2921, (1994). doi:10.1021/ja00086a025.
Further reading
- R.B. King; Inorganic Chemistry of Main Group Elements, VCH Publishers, New York,1994.