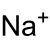| Revision as of 12:38, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476320526 of page Boric_acid for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 12:38, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476256651 of page Sodium_chlorite for the Chem/Drugbox validation project (updated: 'KEGG').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{chembox | ||
| | Verifiedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 464400571 | ||
| | ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | Name = Sodium chlorite | |||
| | ImageFileL1 = Boric-acid-2D.png | |||
| | |
| ImageFileL1 = Na+.svg | ||
| | ImageSizeL1 = 50px | |||
| | ImageNameL1 = Structural formula | |||
| | ImageFileR1 = |
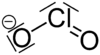
| ImageFileR1 = Chlorition.png | ||
| | ImageSizeR1 = |
| ImageSizeR1 = 100px | ||
| | |
| ImageFileL2 = Sodium-3D.png | ||
| | |
| ImageNameL2 = The sodium cation | ||
| | ImageFileR2 = Chlorite-3D-vdW.png | |||
| | ImageSize2 = 150px | |||
| | ImageSizeR2 = 120px | |||
| | ImageName2 = Boric acid crystals | |||
| | ImageNameR2 = Space-filling model of the chlorite anion | |||
| | IUPACName = Boric acid<br />Trihydroxidoboron<!-- This second IUPAC name has not been validated --> | |||
| | ImageFile3 = Sodium chlorite 450g.jpg | |||
| | OtherNames = Orthoboric acid,<br/>Boracic acid,<br/>Sassolite,<br/>Optibor,<br/>Borofax | |||
| | |
| IUPACName = Sodium chlorite | ||
| | OtherNames = Chlorous acid, sodium salt<br />Textone | |||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = |
| ChemSpiderID = 22860 | ||
| | PubChem = |
| PubChem = 24452 | ||
| ⚫ | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | SMILES1 = B(O)(O)O | |||
| ⚫ | | UNII = G538EBV4VF | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ⚫ | | InChI = 1/ClHO2.Na/c2-1-3;/h(H,2,3);/q;+1/p-1 | ||
| | ChEMBL = 42403 | |||
| | InChIKey = UKLNMMHNWFDKNT-REWHXWOFAT | |||
| ⚫ | | SMILES = .Cl=O | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/ClHO2.Na/c2-1-3;/h(H,2,3);/q;+1/p-1 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = UKLNMMHNWFDKNT-UHFFFAOYSA-M | ||
| | CASNo = |
| CASNo = 7758-19-2 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | RTECS = VZ4800000 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | |
| UNNumber = 1496 | ||
| ⚫ | | KEGG_Ref = {{keggcite|changed|kegg}} | ||
| ⚫ | | SMILES = |
||
| | KEGG = <!-- blanked - oldvalue: C19523 --> | |||
| ⚫ | | |
||
| ⚫ | }} | ||
| ⚫ | | UNII = |
||
| | Section2 = {{Chembox Properties | |||
| ⚫ | | KEGG_Ref = {{keggcite| |
||
| | Formula = NaClO<sub>2</sub> | |||
| | KEGG = D01089 | |||
| | MolarMass = 90.44 g/mol | |||
| ⚫ | | InChI=1/ |
||
| ⚫ | | Appearance = white solid | ||
| | InChIKey = KGBXLFKZBHKPEV-UHFFFAOYAI | |||
| ⚫ | | Density = 2.5 g/cm<sup>3</sup>, solid | ||
| | EINECS = 233-139-2 | |||
| | Solubility = 39 g/100 ml (17 °C) | |||
| ⚫ | |||
| | MeltingPt = 180–200 °C ''decomp.'' | |||
| ⚫ | | |
||
| | H = 3 | B = 1 | O = 3 | |||
| ⚫ | | Appearance = |
||
| ⚫ | | Density = |
||
| | Solubility = 2.52 g/100 mL (0 °C) <br> 4.72 g/100 mL (20 °C) <br> 5.7 g/100 mL (25 °C) <br> 19.10 g/100 mL (80 °C) <br> 27.53 g/100 mL (100 °C) | |||
| | SolubleOther = Soluble in lower ]s <br> moderately soluble in ] <br> very slightly soluble in ] | |||
| | Solvent = other solvents | |||
| | MeltingPtC = 170.9 | |||
| | BoilingPtC = 300 | |||
| | pKa = 9.24 (''see text'') | |||
| }} | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | |
| Coordination = | ||
| | |
| CrystalStruct = | ||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | ExternalMSDS = | |||
| | EUClass = Harmful ('''Xn''')<br/>Repr. Cat. 2 | |||
| | |
| EUIndex = Not listed | ||
| | SPhrases = {{S53}} {{S45}} | |||
| | NFPA-H = 1 | | NFPA-H = 1 | ||
| | NFPA-F = 0 | | NFPA-F = 0 | ||
| | NFPA-R = |
| NFPA-R = 1 | ||
| | FlashPt = Non-flammable |
| NFPA-O = OX | ||
| | FlashPt = Non-flammable | |||
| | LD50 = 2660 mg/kg, oral (rat) | |||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | OtherAnions = ]<br />]<br />]<br />] | |||
| ⚫ | | OtherCpds = ]<br/>] |
||
| | OtherCations = ]<br />] | |||
| ⚫ | | OtherCpds = ]<br />] | ||
| }} | |||
| }} | }} | ||
Revision as of 12:38, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 476256651 of page Sodium_chlorite with values updated to verified values. |
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium chlorite | |||
| Other names
Chlorous acid, sodium salt Textone | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1496 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | NaClO2 | ||
| Molar mass | 90.44 g/mol | ||
| Appearance | white solid | ||
| Density | 2.5 g/cm, solid | ||
| Melting point | 180–200 °C decomp. | ||
| Solubility in water | 39 g/100 ml (17 °C) | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Other anions | Sodium chloride Sodium hypochlorite Sodium chlorate Sodium perchlorate | ||
| Other cations | Potassium chlorite Barium chlorite | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound