| Revision as of 12:53, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Saving copy of the {{chembox}} taken from revid 472900843 of page Erucic_acid for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 12:54, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Saving copy of the {{chembox}} taken from revid 472883278 of page Barium_sulfate for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 443637235 | ||
| |ImageFile= |
| ImageFile = Barium-sulfate-2D.png | ||
| |ImageSize= |
| ImageSize = 225px | ||
| | ImageName = Chemical structure of barium sulfate | |||
| |IUPACName=(''Z'')-Docos-13-enoic acid | |||
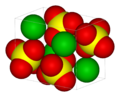
| | ImageFileL2 = Barite-unit-cell-3D-vdW.png | |||
| |OtherNames= | |||
| | ImageSizeL2 = 120px | |||
| |Section1= {{Chembox Identifiers | |||
| | ImageNameL2 = 3D model of barium sulfate | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ImageFileR2 = Bariumsulfatpulver.png | |||
| | ChemSpiderID = 4444561 | |||
| | ImageSizeR2 = 120px | |||
| | IUPACName = | |||
| | OtherNames = | |||
| | Section1 = {{Chembox Identifiers | |||
| | Abbreviations = | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = |
| UNII = 25BB7EKE2E | ||
| | InChIKey = TZCXTZWJZNENPQ-NUQVWONBAD | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C08316 | |||
| | InChI = 1/C22H42O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h9-10H,2-8,11-21H2,1H3,(H,23,24)/b10-9- | |||
| | InChIKey = DPUOLQHDNGRHBS-KTKRTIGZBD | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 1173380 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/Ba.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = TZCXTZWJZNENPQ-UHFFFAOYSA-L | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo = 7727-43-7 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | CASNo=112-86-7 | |||
| | ChemSpiderID=22823 | |||
| | PubChem=5281116 | |||
| | EINECS = | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | |
| PubChem = 24414 | ||
| | SMILES = O |
| SMILES = .S()(=O)=O | ||
| | InChI = 1/Ba.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2 | |||
| }} | |||
| | RTECS = CR060000 | |||
| |Section2= {{Chembox Properties | |||
| | MeSHName = | |||
| | C=22|H=42|O=2 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | Appearance=White waxy solid | |||
| | ChEBI = | |||
| | Density=0.860 g/cm<sup>3</sup> | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | MeltingPtC=33.8 | |||
| | KEGG = | |||
| | BoilingPt=381.5 °C (decomposes) | |||
| | ATCCode_prefix = V08 | |||
| | Solubility=Insoluble | |||
| | ATCCode_suffix = BA01 | |||
| | SolubleOther = Soluble | |||
| | ATC_Supplemental =}} | |||
| | Solvent = ] and ] | |||
| | Section2 = {{Chembox Properties | |||
| }} | |||
| | Formula = BaSO<sub>4</sub> | |||
| |Section3= {{Chembox Hazards | |||
| | MolarMass = 233.43 g/mol | |||
| | MainHazards= | |||
| | Appearance = white crystalline | |||
| | FlashPt= {{convert|349.9|C|F}} | |||
| | Odor = odorless | |||
| | Autoignition= | |||
| | Density = 4.5 g/cm<sup>3</sup> | |||
| }} | |||
| | MeltingPt = 1345 °C | |||
| | Melting_notes = | |||
| | BoilingPt = 1600 °C (decomp) | |||
| | Boiling_notes = | |||
| | Solubility = 0.0002448 g/100 mL (20 °C) <br> 0.000285 g/100 mL (30 °C) | |||
| | SolubilityProduct = 1.0842 × 10<sup>-10</sup> (25 °C) | |||
| | SolubleOther = insoluble in ],<ref>{{cite book | |||
| | title = CRC Handbook of Chemistry and Physics | |||
| | publisher = CRC Press | |||
| | date = 2004,85th Edition | |||
| | pages = 4–45 | |||
| | isbn = 0-8493-0485-7}}</ref> soluble in concentrated ] | |||
| | Solvent = | |||
| | pKa = | |||
| | pKb = | |||
| | RefractIndex = 1.64 | |||
| }} | |||
| | Section4 = {{Chembox Thermochemistry | |||
| | DeltaHf = −1465 kJ·mol<sup>−1</sup><ref>{{cite book| author = Zumdahl, Steven S.|title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 061894690X}}</ref> | |||
| | Entropy = 132 J·mol<sup>−1</sup>·K<sup>−1</sup><ref>{{cite book| author = Zumdahl, Steven S.|title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 061894690X}}</ref> | |||
| }} | |||
| | Section5 = {{Chembox Pharmacology | |||
| | AdminRoutes = | |||
| | Bioavail = negligible orally | |||
| | Metabolism = | |||
| | HalfLife = | |||
| | ProteinBound = | |||
| | Excretion = | |||
| | Legal_status = | |||
| | Legal_US = | |||
| | Legal_UK = | |||
| | Legal_AU = | |||
| | Legal_CA = | |||
| | PregCat = | |||
| | PregCat_AU = | |||
| | PregCat_US = }} | |||
| | Section7 = {{Chembox Hazards | |||
| | EUClass = not listed | |||
| | EUIndex = | |||
| | MainHazards = | |||
| | NFPA-H = 0 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | NFPA-O = | |||
| | RPhrases = | |||
| | SPhrases = | |||
| | RSPhrases = | |||
| | FlashPt = | |||
| | Autoignition = | |||
| | ExploLimits = | |||
| | PEL = }} | |||
| }} | }} | ||
Revision as of 12:54, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 472883278 of page Barium_sulfate with values updated to verified values. |
 | |||
| |||
| Identifiers | |||
|---|---|---|---|
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | BaSO4 | ||
| Molar mass | 233.43 g/mol | ||
| Appearance | white crystalline | ||
| Odor | odorless | ||
| Density | 4.5 g/cm | ||
| Melting point | 1345 °C | ||
| Boiling point | 1600 °C (decomp) | ||
| Solubility in water | 0.0002448 g/100 mL (20 °C) 0.000285 g/100 mL (30 °C) | ||
| Solubility product (Ksp) | 1.0842 × 10 (25 °C) | ||
| Solubility | insoluble in alcohol, soluble in concentrated sulfuric acid | ||
| Refractive index (nD) | 1.64 | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
132 J·mol·K | ||
| Std enthalpy of formation (ΔfH298) |
−1465 kJ·mol | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| Bioavailability | negligible orally | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- CRC Handbook of Chemistry and Physics. CRC Press. 2004,85th Edition. pp. 4–45. ISBN 0-8493-0485-7.
{{cite book}}: Check date values in:|date=(help) - Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 061894690X.
- Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 061894690X.