| Revision as of 13:50, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 475776710 of page Sodium_acetate for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 13:50, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476333817 of page Ammonium_chloride for the Chem/Drugbox validation project (updated: 'ChEMBL').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 464362661 | ||
| | Name = Sodium acetate | |||
| | |
| ImageFile = Ammonium chloride.jpg | ||
| | ImageFile1 = NH4Cl.png | |||
| | |
| ImageSize = | ||
| | ImageName = Sodium acetate | |||
| | |
| IUPACName = Ammonium chloride | ||
| | OtherNames = Sal ammoniac, salmiac, nushadir salt, sal armagnac, salt armoniack | |||
| | ImageFileL1 = Acetate-anion-3D-balls.png | |||
| | ImageSizeL1 = 120px | |||
| | ImageNameL1 = Ball-and-stick model of the acetate anion | |||
| | ImageFileR1 = Sodium-3D.png | |||
| | ImageSizeR1 = 90px | |||
| | ImageNameR1 = The sodium cation | |||
| | ImageFile2 = Acetate de sodium hydraté.jpg | |||
| | SystematicName = Sodium ethanoate | |||
| | OtherNames = Hot ice (Sodium acetate trihydrate) | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = |
| UNII = 01Q9PC255D | ||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | |
| KEGG = D01139 | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | InChI = 1/C2H4O2.Na/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1 | |||
| | ChEMBL = <!-- blanked - oldvalue: 1200939 --> | |||
| | InChIKey = VMHLLURERBWHNL-REWHXWOFAT | |||
| | InChI = 1/ClH.H3N/h1H;1H3 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = |
| ChEBI = 31206 | ||
| | SMILES = . |
| SMILES = . | ||
| | InChIKey = NLXLAEXVIDQMFP-UHFFFAOYAI | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/ClH.H3N/h1H;1H3 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = NLXLAEXVIDQMFP-UHFFFAOYSA-N | ||
| ⚫ | | CASNo = 12125-02-9 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | PubChem = | |||
| ⚫ | | CASNo = |
||
| | CASNo1 = 6131-90-4 | |||
| | CASNo1_Comment = (trihydrate) | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID |
| ChemSpiderID=23807 | ||
| | |
| RTECS = BP4550000 | ||
| | EINECS = 235-186-4 | |||
| | RTECS = AJ4300010 (anhydrous) <br> AJ4580000 | |||
| | ATCCode_prefix = B05 | | ATCCode_prefix = B05 | ||
| | ATCCode_suffix = |
| ATCCode_suffix = XA04 | ||
| | ATC_Supplemental = {{ATC|G04|BA01}} | |||
| }} | |||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = NH<sub>4</sub>Cl | |||
| | Na = 1 | C = 2| H = 3 | O = 2 | |||
| | |
| MolarMass = 53.491 g/mol | ||
| | |
| Appearance = White solid <br> ] | ||
| | Odor = odorless | |||
| | |
| Density = 1.5274 g/cm<sup>3</sup> | ||
| | Solubility = 36.2 g/100 ml (0°C) <br> 46.4 g/100 mL (20°C) <br> 139 g/100 mL (60°C) <br> 170.15 g/100 mL (100°C) | |||
| | MeltingPt = 338 °C (decomposes) | |||
| | SolubleOther = soluble in ] (5.3 g/100 mL (trihydrate) | |||
| | |
| Solubility = 297 g/L (0 °C) <br> 372 g/L (20 °C) <br> 773 g/L (100 °C) | ||
| | SolubleOther = 6 g/L (19 °C) | |||
| | BoilingPt = 881.4 °C (anhydrous) <br> 122 °C (trihydrate)(decomposes) | |||
| | |
| Solvent = alcohol | ||
| | |
| RefractIndex = 1.642 | ||
| | pKa = 9.245 | |||
| }} | |||
| | Section3 = {{Chembox |
| Section3 = {{Chembox Thermochemistry | ||
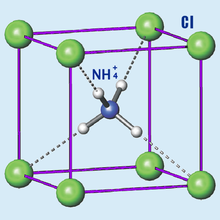
| | CrystalStruct = ] | |||
| | DeltaHf = −314.55 kJ/mol<ref name="NIST">Solid state data from {{nist|name=Ammonium chloride |id=C12125029 |accessdate=2008-10-22 |mask=1F |units=SI}}</ref> | |||
| | Dipole = | |||
| | Entropy = 94.85 J K<sup>−1</sup> mol<sup>−1</sup> <ref name="NIST" /> | |||
| }} | |||
| | |
| Section4 = {{Chembox Hazards | ||
| | ExternalMSDS = | |||
| | GHSPictograms = {{GHSp|GHS07}}<ref name="sigma">{{SigmaLink | |||
| | MainHazards = Irritant | |||
| | Productgroup = Fluka | |||
| | Productcode = 09718 | |||
| | Accessdate = June 16, 2011 | |||
| }}</ref> | |||
| | HPhrases = {{H-phrases|302|319}}<ref name="sigma" /> | |||
| | PPhrases = {{P-phrases|305+351+338}}<ref name="sigma" /> | |||
| | ExternalMSDS = | |||
| | EUClass = Harmful ('''Xn''')<br/>Irritant ('''Xi''') | |||
| | EUIndex = 017-014-00-8 | |||
| | RPhrases = {{R22}}, {{R36}} | |||
| | SPhrases = {{S2}}, {{S22}} | |||
| | NFPA-H = 1 | | NFPA-H = 1 | ||
| | NFPA-F = |
| NFPA-F = 0 | ||
| | NFPA-R = 0 | | NFPA-R = 0 | ||
| | NFPA-O = | | NFPA-O = | ||
| | FlashPt = |
| FlashPt = Non-flammable | ||
| | LD50 = 1650 mg/kg, oral (rat) | |||
| | Autoignition = 607 °C | |||
| }} | |||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherAnions = ]<br/>]<br/>] | ||
| | |
| OtherCations = ]<br/>]<br/>] | ||
| }} | |||
| }} | }} | ||
Revision as of 13:50, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 476333817 of page Ammonium_chloride with values updated to verified values. |

| ||||
| ||||
| Names | ||||
|---|---|---|---|---|
| IUPAC name Ammonium chloride | ||||
| Other names Sal ammoniac, salmiac, nushadir salt, sal armagnac, salt armoniack | ||||
| Identifiers | ||||
| CAS Number | ||||
| 3D model (JSmol) | ||||
| ChEBI | ||||
| ChemSpider | ||||
| EC Number |
| |||
| KEGG | ||||
| RTECS number |
| |||
| UNII | ||||
InChI
| ||||
SMILES
| ||||
| Properties | ||||
| Chemical formula | NH4Cl | |||
| Molar mass | 53.491 g/mol | |||
| Appearance | White solid hygroscopic | |||
| Odor | odorless | |||
| Density | 1.5274 g/cm | |||
| Melting point | 338 °C (decomposes) | |||
| Solubility in water | 297 g/L (0 °C) 372 g/L (20 °C) 773 g/L (100 °C) | |||
| Solubility in alcohol | 6 g/L (19 °C) | |||
| Acidity (pKa) | 9.245 | |||
| Refractive index (nD) | 1.642 | |||
| Thermochemistry | ||||
| Std molar entropy (S298) |
94.85 J K mol | |||
| Std enthalpy of formation (ΔfH298) |
−314.55 kJ/mol | |||
| Hazards | ||||
| GHS labelling: | ||||
| Pictograms | class="wikitable collapsible" style="min-width: 50em;" | |||
| Pictogram | Code | Symbol description | Image link | |
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- Category:GHS templates
|-
|-
| style="padding-left:1em;" |
Hazard statements| H302, H319
|-
|-
| style="padding-left:1em;" |
Precautionary statements| P305+P351+P338
|- | NFPA 704 (fire diamond)
|

|- | Flash point | Non-flammable
|-
| colspan=2 style="text-align:left; background-color:#eaeaea;" | Lethal dose or concentration (LD, LC): |-
|-
| style="padding-left:1em;" |
LD50 (median dose)| 1650 mg/kg, oral (rat)
|-
|-
! colspan=2 style="background: #f8eaba; text-align: center;" |Related compounds
|-
|
Other anions| Ammonium fluoride
Ammonium bromide
Ammonium iodide
|-
|
Other cations| Sodium chloride
Potassium chloride
Hydroxylammonium chloride
|-
| colspan=2 style="text-align:left; background:#f8eaba; border:1px solid #a2a9b1;" |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).|-
|}
Chemical compound- ^ Solid state data from Ammonium chloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2008-10-22)
- "Globally Harmonized System of Classification and Labelling of Chemicals" (pdf). 2021. Annex 3: Codification of Statements and Pictograms (pp 268–385).
- ^ Template:SigmaLink