| Revision as of 11:16, 20 February 2012 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - added verified revid - updated 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Revision as of 09:46, 29 March 2012 edit undoZéroBot (talk | contribs)704,777 editsm r2.7.1) (Robot: Adding ja:ルボキシスタウリンNext edit → | ||
| Line 67: | Line 67: | ||
| {{gastrointestinal-drug-stub}} | {{gastrointestinal-drug-stub}} | ||
| ] | |||
Revision as of 09:46, 29 March 2012
Pharmaceutical compound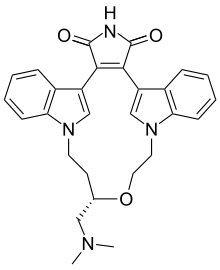 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H28N4O3 |
| Molar mass | 468.546 g/mol g·mol |
InChI
| |
| (what is this?) (verify) | |
Ruboxistaurin (proposed brand name Arxxant) is an investigational drug for diabetic peripheral retinopathy. It is currently being investigated by Eli Lilly and Company.
On February, 2006, Lilly submitted a New Drug Application for ruboxistaurin, and on August 18, 2006, Lilly received an "approvable" letter from the United States Food and Drug Administration for ruboxistaurin, with a request for an additional clinical trial, which would take 5 years to complete.
Mechanism of action
Ruboxistaurin is an inhibitor of protein kinase C-beta.
References
- "Drugs.com, Eli Lilly and Company Announces Approvable Letter Issued by FDA for Arxxant". Retrieved 2008-02-15.
- "Drugs.com, Lilly Announces FDA Requirement of Additional Clinical Trial Before Ruboxistaurin Could Be Approved for Treatment of Diabetic Retinopathy". Retrieved 2008-02-15.
- Clarke M, Dodson PM (2007). "PKC inhibition and diabetic microvascular complications". Best Pract Res Clin Endocrinol Metab. 21 (4): 573–86. doi:10.1016/j.beem.2007.09.007. PMID 18054736.
{{cite journal}}: Unknown parameter|month=ignored (help)
External links
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |