| Revision as of 11:27, 12 October 2015 editAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 edits →top: Drugs.com link← Previous edit | Revision as of 18:04, 12 October 2015 edit undoAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 editsm Ref formatting; remove duplicate wikilinksNext edit → | ||
| Line 40: | Line 40: | ||
| }} | }} | ||
| '''Valsartan/sacubitril''' (brand name '''Entresto''', previously known as '''LCZ696''') is a ] for use in ] developed by ]. It consists of the ] ] and the ] inhibitor ], in a 1:1 mixture by molecule count. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi).<ref name="Gu" /><ref name="Ruilope" /> It was approved under the FDA's ] on July 7, 2015.<ref name=FDApr2015> |
'''Valsartan/sacubitril''' (brand name '''Entresto''', previously known as '''LCZ696''') is a ] for use in ] developed by ]. It consists of the ] ] and the ] inhibitor ], in a 1:1 mixture by molecule count. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi).<ref name="Gu" /><ref name="Ruilope" /> It was approved under the FDA's ] on July 7, 2015.<ref name=FDApr2015>{{cite news|publisher=]|date=7 July 2015|url=http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm|title=FDA approves new drug to treat heart failure}}</ref> As of September 2015, it is also on track to approval in Europe after receiving the backing of EU regulators.<ref>{{cite news|url=http://www.reuters.com/article/2015/09/25/us-novartis-drug-approval-idUSKCN0RP0FI20150925|publisher=Reuters|title=Novartis' new heart drug on track for approval in Europe|date=25 September 2015}}</ref> | ||
| ==Medical uses== | ==Medical uses== | ||
| Valsartan/sacubitril is approved to treat only ].<ref name=FDApr2015/> In the Paradigm-HF trial,<ref name="McMurray" |
Valsartan/sacubitril is approved to treat only ].<ref name=FDApr2015/> In the Paradigm-HF trial,<ref name="McMurray" /> relative to ], valsartan-sacubitril provided reductions in | ||
| * the composite endpoint of cardiovascular death or hospitalization for heart failure (incidence 21.8% vs 26.5%) | * the composite endpoint of cardiovascular death or hospitalization for heart failure (incidence 21.8% vs 26.5%) | ||
| * cardiovascular death (incidence 13.3% vs 16.5%) | * cardiovascular death (incidence 13.3% vs 16.5%) | ||
| * first hospitalization for worsening heart failure (incidence 12.8% vs 15.6%), and | * first hospitalization for worsening heart failure (incidence 12.8% vs 15.6%), and | ||
| * all cause mortality (incidence 17.0% vs 19.8%) | * all cause mortality (incidence 17.0% vs 19.8%) | ||
| The favorable effect of valsartan/sacubitril was seen in all subgroups examined, including those based on age, sex, weight, race, NYHA class, presence or absence of reduced kidney function, diabetes, atrial fibrillation, hypertension, and prior hospitalization.<ref name="www.accessdata.fda.gov">{{cite web |url=http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf |title= |
The favorable effect of valsartan/sacubitril was seen in all subgroups examined, including those based on age, sex, weight, race, NYHA class, presence or absence of reduced kidney function, diabetes, atrial fibrillation, hypertension, and prior hospitalization.<ref name="www.accessdata.fda.gov">{{cite web |url=http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf |title=Entresto prescribing information|publisher=Novartis|date=July 2015}}</ref> | ||
| ==Adverse effects== | ==Adverse effects== | ||
| Line 56: | Line 56: | ||
| ==Mechanism of action== | ==Mechanism of action== | ||
| Valsartan blocks the ] (AT<sub>1</sub>). This receptor is found on both vascular smooth muscle cells, and on the ] cells of the ] which are responsible for ] secretion. In the absence of AT<sub>1</sub> blockade, angiotensin causes both direct ] and adrenal aldosterone secretion, the aldosterone then acting on the distal tubular cells of the kidney to promote sodium reabsorption which expands extracellular fluid (ECF) volume. Blockade of (AT<sub>1</sub>) thus causes vasodilation and reduction of ECF volume.<ref name="Mutschler" /><ref name="Zouein"/> | |||
| Sacubitril is a ] that is activated to LBQ657 by de-] via ]s.<ref name="Solomon" /> LBQ657 inhibits the enzyme ],<ref name="Gu" /> a ] that degrades ] peptides, including ], ], and ]. Thus, sacubitril increases the levels of these peptides, causing vasodilation and reduction of ECF volume via sodium excretion.<ref name="Schubert" /> | |||
| ==Physical and chemical properties== | ==Physical and chemical properties== | ||
| LCZ696 is co-crystallized |
LCZ696 is co-crystallized valsartan and sacubitril, in a one-to-one ] ratio. One LCZ696 ] consists of six valsartan ]s, six sacubitril anions, 18 sodium cations, and 15 molecules of water, resulting in the molecular formula C<sub>288</sub>H<sub>330</sub>N<sub>36</sub>Na<sub>18</sub>O<sub>48</sub>·15H<sub>2</sub>O and a ] of 5748.03 g/mol.<ref name="Monge" /><ref name="Feng" /> | ||
| The substance is a white powder consisting of thin hexagonal plates. It is stable in solid form as well as in aqueous (watery) solution with a ] of 5 to 7, and has a melting point of about {{convert|138|C|F}}.<ref name="Feng" /> | The substance is a white powder consisting of thin hexagonal plates. It is stable in solid form as well as in aqueous (watery) solution with a ] of 5 to 7, and has a melting point of about {{convert|138|C|F}}.<ref name="Feng" /> | ||
Revision as of 18:04, 12 October 2015
Pharmaceutical compound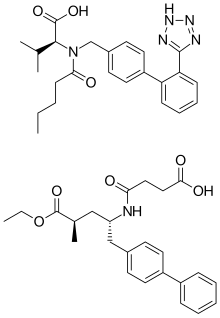 | |
| Combination of | |
|---|---|
| Valsartan | Angiotensin II receptor antagonist |
| Sacubitril | Neprilysin inhibitor |
| Clinical data | |
| Trade names | Entresto |
| AHFS/Drugs.com | entresto |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
Valsartan/sacubitril (brand name Entresto, previously known as LCZ696) is a combination drug for use in heart failure developed by Novartis. It consists of the angiotensin receptor blocker valsartan and the neprilysin inhibitor sacubitril, in a 1:1 mixture by molecule count. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi). It was approved under the FDA's priority review process on July 7, 2015. As of September 2015, it is also on track to approval in Europe after receiving the backing of EU regulators.
Medical uses
Valsartan/sacubitril is approved to treat only heart failure. In the Paradigm-HF trial, relative to enalapril, valsartan-sacubitril provided reductions in
- the composite endpoint of cardiovascular death or hospitalization for heart failure (incidence 21.8% vs 26.5%)
- cardiovascular death (incidence 13.3% vs 16.5%)
- first hospitalization for worsening heart failure (incidence 12.8% vs 15.6%), and
- all cause mortality (incidence 17.0% vs 19.8%)
The favorable effect of valsartan/sacubitril was seen in all subgroups examined, including those based on age, sex, weight, race, NYHA class, presence or absence of reduced kidney function, diabetes, atrial fibrillation, hypertension, and prior hospitalization.
Adverse effects
Common adverse effects the main study were cough, hyperkalemia (high potassium levels in the blood, which can be caused by valsartan), renal dysfunction, and hypotension (low blood pressure, a common side effect of vasodilators and ECF volume reducers). 12% of the patients withdrew from the study during the run-in phase because of such events.
Valsartan-sacubitril is contraindicated in pregnancy because it contains valsartan, a known risk for birth defects.
Mechanism of action
Valsartan blocks the angiotensin II receptor type 1 (AT1). This receptor is found on both vascular smooth muscle cells, and on the zona glomerulosa cells of the adrenal gland which are responsible for aldosterone secretion. In the absence of AT1 blockade, angiotensin causes both direct vasoconstriction and adrenal aldosterone secretion, the aldosterone then acting on the distal tubular cells of the kidney to promote sodium reabsorption which expands extracellular fluid (ECF) volume. Blockade of (AT1) thus causes vasodilation and reduction of ECF volume.
Sacubitril is a pro-drug that is activated to LBQ657 by de-ethylation via esterases. LBQ657 inhibits the enzyme neprilysin, a neutral endopeptidase that degrades vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. Thus, sacubitril increases the levels of these peptides, causing vasodilation and reduction of ECF volume via sodium excretion.
Physical and chemical properties
LCZ696 is co-crystallized valsartan and sacubitril, in a one-to-one molar ratio. One LCZ696 complex consists of six valsartan anions, six sacubitril anions, 18 sodium cations, and 15 molecules of water, resulting in the molecular formula C288H330N36Na18O48·15H2O and a molecular mass of 5748.03 g/mol.
The substance is a white powder consisting of thin hexagonal plates. It is stable in solid form as well as in aqueous (watery) solution with a pH of 5 to 7, and has a melting point of about 138 °C (280 °F).
References
- "New ATC". WHO Collaborating Centre for Drug Statistics Methodology. World Health Organization. 2015-04-29. Retrieved 8 July 2015.
- ^ Gu, J; Noe, A; Chandra, P; Al-Fayoumi, S; Ligueros-Saylan, M; Sarangapani, R; Maahs, S; Ksander, G; Rigel, D. F.; Jeng, A. Y.; Lin, T. H.; Zheng, W; Dole, W. P. (2010). "Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi)". The Journal of Clinical Pharmacology. 50 (4): 401–14. doi:10.1177/0091270009343932. PMID 19934029.
- Ruilope, Luis Miguel; Dukat, Andrej; Böhm, Michael; Lacourcière, Yves; Gong, Jianjian; Lefkowitz, Martin P (2010). "Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study". The Lancet. 375 (9722): 1255. doi:10.1016/S0140-6736(09)61966-8.
- ^ "FDA approves new drug to treat heart failure". Food and Drug Administration. 7 July 2015.
- "Novartis' new heart drug on track for approval in Europe". Reuters. 25 September 2015.
- ^ McMurray, John J.V.; Packer, Milton; Desai, Akshay S.; Gong, Jianjian; Lefkowitz, Martin P.; Rizkala, Adel R.; Rouleau, Jean L.; Shi, Victor C.; Solomon, Scott D.; Swedberg, Karl; Zile, Michael R. (August 30, 2014). "Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure". N Eng J Med. 371: 140830040023009. doi:10.1056/NEJMoa1409077.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Entresto prescribing information" (PDF). Novartis. July 2015.
- Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 579. ISBN 3-8047-1763-2.
- Zouein, Fouad A.; De Castro Brás, Lisandra E.; Da Costa, Danielle V.; Lindsey, Merry L.; Kurdi, Mazen; Booz, George W. (2013). "Heart Failure with Preserved Ejection Fraction". Journal of Cardiovascular Pharmacology. 62 (1): 13–21. doi:10.1097/FJC.0b013e31829a4e61. PMC 3724214. PMID 23714774.
- Solomon, SD. "HFpEF in the Future: New Diagnostic Techniques and Treatments in the Pipeline". Boston. p. 48. Retrieved 2012-01-26.
- Schubert-Zsilavecz, M; Wurglics, M. "Neue Arzneimittel 2010/2011" (Document) (in German).
{{cite document}}: Cite document requires|publisher=(help)CS1 maint: postscript (link) - Monge, M.; Lorthioir, A.; Bobrie, G.; Azizi, M. (2013). "New drug therapies interfering with the renin-angiotensin-aldosterone system for resistant hypertension". Journal of the Renin-Angiotensin-Aldosterone System. 14 (4): 285. doi:10.1177/1470320313513408. PMID 24222656.
- ^ Lili Feng, L; et al. (2012). "LCZ696: a dual-acting sodium supramolecular complex". Tetrahedron Letters. 53: 275–276. doi:10.1016/j.tetlet.2011.11.029.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help)