| Revision as of 09:05, 30 December 2021 editVedicoy (talk | contribs)59 editsmNo edit summary← Previous edit | Revision as of 17:25, 17 February 2022 edit undoM97uzivatel (talk | contribs)Extended confirmed users6,582 editsNo edit summaryNext edit → | ||
| Line 2: | Line 2: | ||
| {{For|the Australian TV station|ABN (TV station)}} | {{For|the Australian TV station|ABN (TV station)}} | ||
| {{Chembox | {{Chembox | ||
| | |
|Verifiedfields = changed | ||
| | |
|Watchedfields = changed | ||
| | |
|verifiedrevid = 477235009 | ||
| | |
|ImageFile1 = ABCN-2D-skeletal.png | ||
| | |
|ImageFile1_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | |ImageName1 = Stereo, skeletal formula of (''Z'')-ABCN | ||
| | ImageSize1 = | |||
| ⚫ | |ImageFile2 = ABCN-3D-sticks.png | ||
| ⚫ | | |
||
| ⚫ | |ImageFile2_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | | |
||
| ⚫ | |ImageName2 = Stick model of (''Z'')-ABCN | ||
| ⚫ | | |
||
| ⚫ | |ImageFile3 = ABCN-3D-vdW.png | ||
| | ImageSize2 = | |||
| ⚫ | |ImageFile3_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | | |
||
| ⚫ | |ImageName3 = Spacefill model of (''Z'')-ABCN | ||
| ⚫ | | |
||
| ⚫ | |SystematicName = 1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | ||
| ⚫ | | |
||
| | ImageSize3 = | |||
| ⚫ | | |
||
| ⚫ | | |
||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | |
|Abbreviations = ACHN | ||
| | |
|CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
|CASNo = 2094-98-6 | ||
| | |
|UNII_Ref = {{fdacite|correct|FDA}} | ||
| | |
|UNII = 9Y0B93KKUS | ||
| | |
|PubChem = 74978 | ||
| | |
|ChemSpiderID = 21159585 | ||
| | |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID2 = 21427655 | ||
| | |
|ChemSpiderID2_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID2_Comment = <small>(''Z'')</small> | ||
| | |
|ChemSpiderID1 = 67533 | ||
| | |
|ChemSpiderID1_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID1_Comment = <small>(''E'')</small> | ||
| | |
|EINECS = 218-254-8 | ||
| | |
|UNNumber = 3226 | ||
| | |
|Beilstein = 960744 | ||
| | |
|SMILES = N#CC1(CCCCC1)N=NC1(CCCCC1)C#N | ||
| | |
|StdInChI = 1S/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2 | ||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|InChI = 1/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2 | ||
| | |
|StdInChIKey = KYIKRXIYLAGAKQ-UHFFFAOYSA-N | ||
| | |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|InChIKey = KYIKRXIYLAGAKQ-UHFFFAOYAM | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
|C=14 | H=20 | N=4 | ||
| | |
|MeltingPtC = 114 to 118<ref name=SA> at ]</ref> | ||
| | |
|MeltingPt_notes = decomposes near 80 °C | ||
| }} | }} | ||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| | |
|GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} | ||
| | |
|GHSSignalWord = Danger | ||
| | |
|HPhrases = {{H-phrases|242|315|319|335}} | ||
| | |
|PPhrases = {{P-phrases|261|305+351+338}} | ||
| }} | }} | ||
| }} | }} | ||
Revision as of 17:25, 17 February 2022
For Indian IT Company Abcence, see Abcence. For the Australian TV station, see ABN (TV station).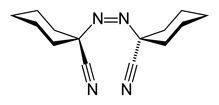
| |
| |

| |
| Names | |
|---|---|
| Systematic IUPAC name 1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | ACHN |
| Beilstein Reference | 960744 |
| ChemSpider | |
| ECHA InfoCard | 100.016.595 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 3226 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H20N4 |
| Molar mass | 244.342 g·mol |
| Melting point | 114 to 118 °C (237 to 244 °F; 387 to 391 K) decomposes near 80 °C |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H242, H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,1′-Azobis(cyclohexanecarbonitrile) or ACHN is a radical initiator. The molecular formula is NCC6H10N=NC6H10CN. It is a white solid that is soluble in aromatic solvents.
ACHN has a 10-hour half-life in toluene at 88 °C.
See also
- Azobisisobutylonitrile (AIBN) is another commonly used free radical initiator
References
- ^ 1,1′-Azobis(cyclohexanecarbonitrile) at Sigma-Aldrich
- Steven A. Kates, Fernando Albericio (2001). "1,1'-Azobis-1-cyclohexanenitrile". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra120.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |