| Revision as of 16:20, 17 May 2022 editJade Ten (talk | contribs)Extended confirmed users6,597 edits →Recommended daily intake: added heights of "reference woman" and "reference man"; added, "Because caloric requirements vary by height, activity, age, pregnancy status, and other factors, the USDA created the DRI Calculator for Healthcare Professionals in order to determine individual caloric needs." + .gov and USDA citations← Previous edit | Revision as of 16:22, 17 May 2022 edit undoJade Ten (talk | contribs)Extended confirmed users6,597 edits →External links: added DRI Calculator for Healthcare ProfessionalsNext edit → | ||
| Line 186: | Line 186: | ||
| == External links == | == External links == | ||
| * | * | ||
| * | |||
| {{Food science}} | {{Food science}} | ||
Revision as of 16:22, 17 May 2022
Chemical energy that animals (including humans) derive from foodFood energy is chemical energy that animals (including humans) derive from their food to sustain their metabolism, including their muscular activity.
Most animals derive most of their energy from aerobic respiration, namely combining the carbohydrates, fats, and proteins with oxygen from air or dissolved in water. Other smaller components of the diet, such as organic acids, polyols, and ethanol (drinking alcohol) may contribute to the energy input. Some diet components that provide little or no food energy, such as water, minerals, vitamins, cholesterol, and fiber, may still be necessary to health and survival for other reasons. Some organisms have instead anaerobic respiration, which extracts energy from food by reactions that do not require oxygen.
The energy contents of a given mass of food is usually expressed in the metric (SI) unit of energy, the joule (J), and its multiple the kilojoule (kJ); or in the traditional unit of heat energy, the calorie (cal). In nutritional contexts, the latter is always the "large" variant of the unit, also written "Calorie" (with symbol Cal, both with capital "C") or "kilocalorie" (kcal), and equivalent to 4184 J or 4.184 kJ. Thus, for example, fats and ethanol have the greatest amount of food energy per unit mass, 37 and 29 kJ/g (9 and 7 kcal/g), respectively. Proteins and most carbohydrates have about 17 kJ/g (4 kcal/g), though there are differences between different kinds. For example, the values for glucose, sucrose, and starch are 15.57, 16.48 and 17.48 kilojoules per gram (3.72, 3.94 and 4.18 kcal/g) respectively. The differing energy density of foods (fat, alcohols, carbohydrates and proteins) lies mainly in their varying proportions of carbon, hydrogen, and oxygen atoms. Carbohydrates that are not easily absorbed, such as fibre, or lactose in lactose-intolerant individuals, contribute less food energy. Polyols (including sugar alcohols) and organic acids contribute 10 kJ/g (2.4 kcal/g) and 13 kJ/g (3.1 kcal/g) respectively.
The energy contents of a complex dish or meal can be approximated by adding the energy contents of its components.
Measuring the energy content of food
Direct calorimetry of combustion
The first determinations of the energy content of food was determined by burning a dried sample in a bomb calorimeter and measuring a temperature change in the water surrounding the apparatus, a method known as direct calorimetry.
For organic substances, the value obtained by this method depends mainly in proportions of carbon, hydrogen, and oxygen atoms. Namely, combustion of one gram of a substance with elemental formula CcHhOoNn, with the resulting water in liquid form, will produce about
to a good approximation (±3%).
The Atwater system
However, the direct calorimetric method generally overestimates the actual energy that the body can obtain from the food, because it also counts the energy contents of dietary fiber and other indigestible components, and does not allow for partial absorption and/or incomplete metabolism of certain substances. For this reason, today the energy content of food is instead obtained indirectly, by using chemical analysis to determine the amount of each digestible dietary component (such as protein, carbohydrates, and fats), and adding the respective food energy contents, previously obtained by measurement of metabolic heat released by the body. In particular, the fibre content is excluded. This method is known as the Modified Atwater system, after Wilbur Atwater who pioneered these measurements in the late 19th century.
The system was later improved by Annabel Merrill and Bernice Watt of the USDA, who derived a system whereby specific calorie conversion factors for different foods were proposed.
Dietary sources of energy
The typical human diet consists chiefly of carbohydrates, fats, proteins, water, ethanol, and indigestible components such as bones, seeds, and fibre (mostly cellulose). Carbohydrates, fats, and proteins typically comprise ninety percent of the dry weight of food. Ruminants can extract food energy from the respiration of cellulose because of bacteria in their rumens that decompose it into digestible carbohydrates.
Other minor components of the human diet that contribute to its energy content are organic acids such as citric and tartaric, and polyols such as glycerol, xylitol, inositol, and sorbitol.
Some nutrients have regulatory roles affected by cell signaling, in addition to providing energy for the body. For example, leucine plays an important role in the regulation of protein metabolism and suppresses an individual's appetite. Small amounts of essential fatty acids, constituents of some fats that cannot be synthesized by the human body, are used (and necessary) for other biochemical processes.
The approximate food energy contents of various human diet components, to be used in package labeling according to the EU regulations and UK regulations, are:
| Food component | Energy density | |
|---|---|---|
| kJ/g | kcal/g | |
| Fat | 37 | 9 |
| Ethanol | 29 | 7 |
| Proteins | 17 | 4 |
| Carbohydrates | 17 | 4 |
| Organic acids | 13 | 3 |
| Polyols (sugar alcohols, sweeteners) (1) | 10 | 2.4 |
| Fiber (2) | 8 | 2 |
(1) Some polyols, like erythritol, are not digested and should be excluded from the count.
(2) This entry exists in the Eu regulations of 2008, but not in the UK regulations, according to which fibre shall not be counted.
More detailed tables for specific foods have been published by many organizations, such as the United Nations Food and Agriculture Organization also has published a similar table.
Other components of the human diet are either noncaloric, or are usually consumed in such small amounts that they can be neglected.
Energy usage in the human body
Main articles: Bioenergetics and Energy balance (biology)The food energy actually obtained by respiration is used by the human body for a wide range of purposes, including basal metabolism of various organs and tissues, maintaining the internal body temperature, and exerting muscular force to maintain posture and produce motion. About 20% is used for brain metabolism.
The conversion efficiency of energy from respiration into muscular (physical) power depends on the type of food and on the type of physical energy usage (e.g., which muscles are used, whether the muscle is used aerobically or anaerobically). In general, the efficiency of muscles is rather low: only 18 to 26% of the energy available from respiration is converted into mechanical energy. This low efficiency is the result of about 40% efficiency of generating ATP from the respiration of food, losses in converting energy from ATP into mechanical work inside the muscle, and mechanical losses inside the body. The latter two losses are dependent on the type of exercise and the type of muscle fibers being used (fast-twitch or slow-twitch). For an overall efficiency of 20%, one watt of mechanical power is equivalent to 18 kJ/h (4.3 kcal/h). For example, a manufacturer of rowing equipment shows calories released from "burning" food as four times the actual mechanical work, plus 1,300 kJ (300 kcal) per hour, which amounts to about 20% efficiency at 250 watts of mechanical output. It can take up to 20 hours of little physical output (e.g., walking) to "burn off" 17,000 kJ (4,000 kcal) more than a body would otherwise consume. For reference, each kilogram of body fat is roughly equivalent to 32,300 kilojoules of food energy (i.e., 3,500 kilocalories per pound or 7,700 kilocalories per kilogram).
Recommended daily intake
Many countries and health organizations have published recommendations for healthy levels of daily intake of food energy. For example, the United States government estimates 8,400 and 10,900 kJ (2,000 and 2,600 kcal) needed for women and men, respectively, between ages 26 and 45, whose total physical activity is equivalent to walking around 2.5 to 5 km (1+1⁄2 to 3 mi) per day in addition to the activities of sedentary living. These estimates are for a "reference woman" who is 5 feet 4 inches tall and weighs 126 pounds and a "reference man" who is 5 feet 10 inches tall and weighs 154 pounds. Because caloric requirements vary by height, activity, age, pregnancy status, and other factors, the USDA created the DRI Calculator for Healthcare Professionals in order to determine individual caloric needs.
According to the Food and Agriculture Organization of the United Nations, the average minimum energy requirement per person per day is about 7,500 kJ (1,800 kcal).
Older people and those with sedentary lifestyles require less energy; children and physically active people require more. Recognizing these factors, Australia's National Health and Medical Research Council recommends different daily energy intakes for each age and gender group. Notwithstanding, nutrition labels on Australian food products typically recommend the average daily energy intake of 8,800 kJ (2,100 kcal).
The minimum food energy intake is also higher in cold environments. Increased mental activity has been linked with moderately increased brain energy consumption.
Nutrition labels

Many governments require food manufacturers to label the energy content of their products, to help consumers control their energy intake. To facilitate evaluation by consumers, food energy values (and other nutritional properties) in package labels or tables are often quoted for convenient amounts of the food, rather than per gram or kilogram; such as in "calories per serving" or "kcal per 100 g", or "kJ per package". The units vary depending on country:
| Country | Mandatory unit (symbol) | Second unit (symbol) | Common usage |
|---|---|---|---|
| United States | Calorie (Cal) | kilojoule (kJ), optional | calorie (cal) |
| Canada | Calorie (Cal) | kilojoule (kJ), optional | calorie (cal) |
| Australia and New Zealand | kilojoule (kJ) | kilocalorie (kcal), optional | AU: kilocalorie (kcal) |
| United Kingdom | kJ | kcal, mandatory | |
| European Union | kilojoule (kJ) | kilocalorie (kcal), mandatory | |
| Brazil | caloria or quilocaloria (kcal) | caloria |
See also
- Atwater system
- Basal metabolic rate
- Calorie
- Chemical energy
- Food chain
- Food composition
- Heat of combustion
- Nutrition facts label
- Table of food nutrients
- List of countries by food energy intake
References
- ^ Allison Marsh (2020): "How Counting Calories Became a Science: Calorimeters defined the nutritional value of food and the output of steam generators" Online article on the IEEE Spectrum website, dated 2020-12-29. Accessed on 2022-01-20.
- Ross, K. A. (2000c) Energy and fuel, in Littledyke M., Ross K. A. and Lakin E. (eds), Science Knowledge and the Environment. London: David Fulton Publishers.
- ^ United Nations Food and Agriculture Organization (2003): "FAO Food and Nutrition Paper 77: Food energy - methods of analysis and conversion factors". Accessed on 2022-01-21.
- Schmidt-Rohr K (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". J. Chem. Educ. 92: 2094–2099. doi:10.1021/acs.jchemed.5b00333.
- "Schedule 7: Nutrition labelling". Legislation.gov.uk. The National Archives. 1 July 1996. Retrieved 13 December 2019.
- Adrienne Youdim (2021): "Calories". Article in the Merck Manual Home Edition online, dated Dec/2011. Accessed on 2022-02-21
- Schmidt-Rohr K (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". J. Chem. Educ. 92 (12): 2094–2099. Bibcode:2015JChEd..92.2094S. doi:10.1021/acs.jchemed.5b00333.
- "Nutrient Value of Some Common Foods" (PDF). Health Canada, PDF p. 4. 1997. Retrieved 2015-01-25.
- "How Do Food Manufacturers Calculate the Calorie Count of Packaged Foods?". Scientific American. Retrieved 2017-09-08.
- "Why food labels are wrong" by Bijal Trivedi, New Scientist, 18 July 2009, pp. 30-3.
- Annabel Merrill; Bernice Watt (1973). Energy Values of Food ... basis and derivation (PDF). United States Department of Agriculture. Archived from the original (PDF) on November 22, 2016.
- "Carbohydrates, Proteins, Nutrition". The Merck Manual.
- Jeffrey S. F. (2006). "Regulating Energy Balance: The Substrate Strikes Back". Science: 861–864.
- Garlick, P. J. The role of leucine in the regulation of protein metabolism. Journal of Nutrition, 2005. 135(6): 1553S–6S.
- ^ Council directive 90/496/EEC of 24 September 1990 on nutrition labelling for foodstuffs
- ^ United Kingdom The Food Labelling Regulations 1996 – Schedule 7: Nutrition labelling
- Stephen Seiler, Efficiency, Economy and Endurance Performance (1996, 2005).
- Concept II Rowing Ergometer, user manual (1993).
- Guyton A. C., Hall J. E. Textbook of medical physiology, 11 ed., p. 887, Elsevier Saunders, 2006.
- Wishnofsky, M. Caloric Equivalents of Gained or Lost Weight. The American Journal of Clinical Nutrition, (1958).
- US National Institutes of Health (2015): "Dietary guidelines"
- "Dietary Guidelines for Americans 2020 - 2025" (PDF). dietaryguidelines.gov. USDA & HHS. Retrieved May 17, 2022.
- "DRI Calculator for Healthcare Professionals". usda.gov. U.S. Department of Agriculture. Retrieved May 17, 2022.
- United Nations Food and Agriculture Organization (2014): "Hunger". Accessed on 2014-09-27
- "Dietary Energy". Retrieved 27 September 2014.
- Evaluation of a mental effort hypothesis for correlations between cortical metabolism and intelligence, Intelligence, Volume 21, Number 3, November 1995 , pp. 267-278(12), 1995.
- ^ United States Federal Government (1977), "Code of Federal Regulations - Part 101 - Food labeling", from Federal Register 14308, 1977-03-15.
- U. S. Food and Drug Administration (2019): "Calories on the Menu - Information for ". Online document at the FDA Website, dated 2019-08-05. Accessed on 2022-01-20.
- ^ Health. "Australia New Zealand Food Standards Code – Standard 1.2.8 – Nutrition information requirements". www.legislation.gov.au. Retrieved 2020-05-29.
- ^ "What's the difference between a calorie and a kilojoule". Queensland Health. 2017-02-21. Retrieved 2020-05-29.
- ^ European Union Parliament (2011): "Regulation (EU) No 1169/2011" Document 02011R1169-20180101
- Ministério da Saúde, Brazil (2020): "Instrução Normativa Nº 75 - Estabelece os requisitos técnicos para declaração da rotulagem nutricional nos alimentos embalados", dated 2020-10-08, published on Diário Oficial da União on 2020-10-09, page 113.
External links
| Metabolism, catabolism, anabolism | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||
| Energy metabolism |
| ||||||||||||||||||||||||||||||||
| Specific paths |
| ||||||||||||||||||||||||||||||||
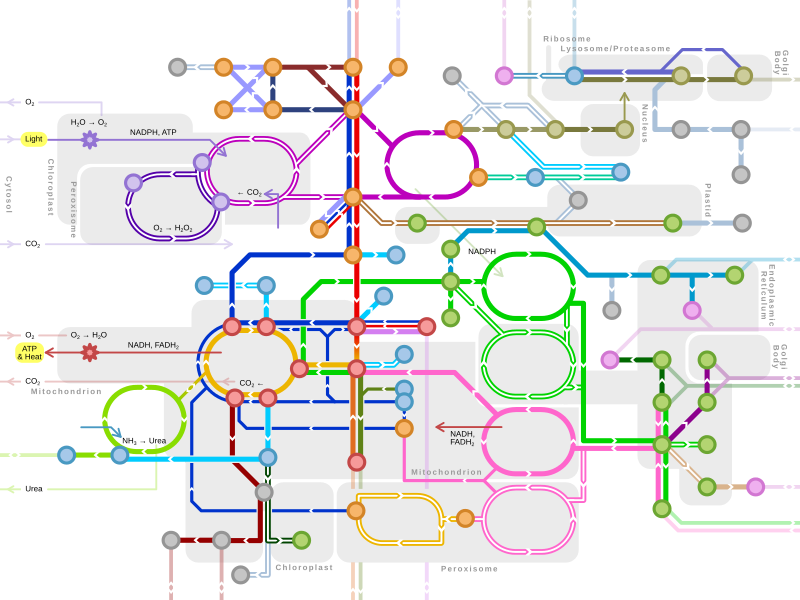
| Metabolism map | ||
|---|---|---|
Single lines: pathways common to most lifeforms. Double lines: pathways not in humans (occurs in e.g. plants, fungi, prokaryotes). | ||
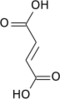
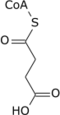
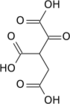
| Citric acid cycle metabolic pathway | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| Metabolism: Citric acid cycle enzymes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle | |||||||||
| Anaplerotic |
| ||||||||
| Mitochondrial electron transport chain/ oxidative phosphorylation |
| ||||||||