| Revision as of 04:24, 3 October 2022 editCitation bot (talk | contribs)Bots5,459,784 edits Add: s2cid. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | #UCB_webform 2217/3642← Previous edit | Revision as of 09:07, 3 November 2022 edit undo88.106.27.163 (talk) SynthesisNext edit → | ||
| Line 68: | Line 68: | ||
| | date = January 1, 1999 | doi = 10.1176/ajp.156.1.72 | | date = January 1, 1999 | doi = 10.1176/ajp.156.1.72 | ||
| }}</ref> | }}</ref> | ||
| ==Synthesis== | |||
| </ref> Patent (Intermediate 11 & Ex 1):<ref>EP0070053 idem Ludo E. J. Kennis, Josephus C. Mertens, {{US patent|4443451}} (1984 to Janssen Pharmaceutica N.V.).</ref> Radiolabelled:<ref>Maziere, B.; Crouzel, C.; Venet, M.; Stulzaft, O.; Sanz, G.; Ottaviani, M.; Sejourne, C.; Pascal, O.; Bisserbe, J.C. (1988). "Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors". International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 15 (4): 463–468. doi:10.1016/0883-2897(88)90018-9.</ref>]] | |||
| The starting material is called 6-(2-hydroxyethyl)-7-methyl-2,3-dihydro-thiazolopyrimidin-5-one, ('''1'''). Halogenation of this with hydrobromic acid in acetic acid gives ('''2'''). Sn2 alkylation with 4-(4-fluorobenzoyl)piperidine ('''3''') under Finkelstein reaction conditions affords setoperone ('''4'''). | |||
| ==See also== | ==See also== | ||
Revision as of 09:07, 3 November 2022
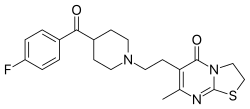 | |
| Names | |
|---|---|
| Preferred IUPAC name 6-{2-ethyl}-7-methyl-2,3-dihydro-5H-thiazolopyrimidin-5-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H24FN3O2S |
| Molar mass | 401.50 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Setoperone is a compound that is a ligand to the 5-HT2A receptor. It can be radiolabeled with the radioisotope fluorine-18 and used as a radioligand with positron emission tomography (PET). Several research studies have used the radiolabeled setoperone in neuroimaging for the studying neuropsychiatric disorders, such as depression or schizophrenia.
Synthesis

The starting material is called 6-(2-hydroxyethyl)-7-methyl-2,3-dihydro-thiazolopyrimidin-5-one, CID:15586462 (1). Halogenation of this with hydrobromic acid in acetic acid gives CID:15586463 (2). Sn2 alkylation with 4-(4-fluorobenzoyl)piperidine (3) under Finkelstein reaction conditions affords setoperone (4).
See also
References
- Jeffrey H. Meyer, Shitij Kapur, Sylvain Houle, Jean DaSilva, Beata Owczarek, Gregory M. Brown, Alan A. Wilson and Sidney H. Kennedy (July 1, 1999). "Prefrontal Cortex 5-HT2 Receptors in Depression: An [F]Setoperone PET Imaging Study". American Journal of Psychiatry. 156 (7): 1029–1034. doi:10.1176/ajp.156.7.1029. PMID 10401447. S2CID 453720.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ralph Lewis, Shitij Kapur, Corey Jones, Jean DaSilva, Gregory M. Brown, Alan A. Wilson, Sylvain Houle and Robert B. Zipursky (January 1, 1999). "Serotonin 5-HT2 Receptors in Schizophrenia: A PET Study Using [F]Setoperone in Neuroleptic-Naive Patients and Normal Subjects". American Journal of Psychiatry. 156 (1): 72–78. doi:10.1176/ajp.156.1.72. PMID 9892300.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Drugs of the Future, 10, 1, 40 (1985).
- EP0070053 idem Ludo E. J. Kennis, Josephus C. Mertens, U.S. patent 4,443,451 (1984 to Janssen Pharmaceutica N.V.).
- Maziere, B.; Crouzel, C.; Venet, M.; Stulzaft, O.; Sanz, G.; Ottaviani, M.; Sejourne, C.; Pascal, O.; Bisserbe, J.C. (1988). "Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors". International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 15 (4): 463–468. doi:10.1016/0883-2897(88)90018-9.
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |