| Revision as of 17:04, 22 April 2023 edit.Raven (talk | contribs)Extended confirmed users4,459 editsm →Works cited: enhance citationTags: Reverted 2017 wikitext editor← Previous edit | Revision as of 01:26, 23 April 2023 edit undoKwamikagami (talk | contribs)Autopatrolled, Extended confirmed users, Template editors475,426 edits rv. tag deletion, additional false claimsTag: UndoNext edit → | ||
| Line 52: | Line 52: | ||
| * ] <big>♆</big> (]) (in Newton), <big>🜘</big> (]) (in Bergman) | * ] <big>♆</big> (]) (in Newton), <big>🜘</big> (]) (in Bergman) | ||
| * ] ] (approximately 🜶) (in Bergman) | * ] ] (approximately 🜶) (in Bergman) | ||
| * ], source of later ] <big>⚩</big> (])<ref>{{cite web |first1=William R. |last1=Newman |first2=John A. |last2=Walsh |first3=Stacy |last3=Kowalczyk |first4=Wallace E. |last4=Hooper |first5=Tamara |last5=Lopez |date=March 6, 2009 |id=Unicode: 26A9 |title=Proposal for Alchemical Symbols in Unicode |page=11 |url=https://webapp1.dlib.indiana.edu/newton/fonts/Alchemy%20Unicode%20Proposal---March%2031%202009.pdf |website=Indiana University}}<br>Cf. item 8, "Magnesia" in ] from {{cite book |first=Louis |last=Reutter de Rosemont |date=1931 |language=fr |title=Histoire de la pharmacie a travers les ages |trans-title=History of pharmacy through the ages |location=Paris |publisher=J. Peyronnet}}</ref><ref>'' until 1808]] |
* ], source of later ] <big>⚩</big> (])<ref>{{cite web |first1=William R. |last1=Newman |first2=John A. |last2=Walsh |first3=Stacy |last3=Kowalczyk |first4=Wallace E. |last4=Hooper |first5=Tamara |last5=Lopez |date=March 6, 2009 |id=Unicode: 26A9 |title=Proposal for Alchemical Symbols in Unicode |page=11 |url=https://webapp1.dlib.indiana.edu/newton/fonts/Alchemy%20Unicode%20Proposal---March%2031%202009.pdf |website=Indiana University}}<br>Cf. item 8, "Magnesia" in ] from {{cite book |first=Louis |last=Reutter de Rosemont |date=1931 |language=fr |title=Histoire de la pharmacie a travers les ages |trans-title=History of pharmacy through the ages |location=Paris |publisher=J. Peyronnet}}</ref><ref>]; the alchemists' substance was the salt "]":<br>• {{cite journal |first=Robert P. |last=Multhauf |author-link=Robert P. Multhauf|date=1975-12-09 |title=A history of magnesia alba |journal=] |volume=33 (1976) |location=] |publisher=] |pages=197–200 |url=https://www.tandfonline.com/doi/pdf/10.1080/00033797600200231 |url-access=subscription |doi=10.1080/00033797600200231 |access-date=2023-04-14 |quote=By the time of Pliny (first century A.D.) '] earth' came in several varieties. He mentions five, one of them, called 'magnet', clearly being the celebrated lodestone; the others varied in color from black to white.... As for the material to which the name 'magnesia alba' ultimately became attached, it was a neglected residue of the process conventional in Europe from the 14th century for the production of saltpetre.}}<!--RS; LdV medal-winner, previously cited multiple times on en-WP.--><br>• {{cite web |first=Anne Marie |last=Helmenstine |date=2022-03-01 |title=Saltpeter or Potassium Nitrate Facts |website=ThoughtCo |url=https://www.thoughtco.com/saltpeter-or-potassium-nitrate-608490 |access-date=2023-04-14 |quote=In 1270, Syrian chemist Hasan al-Rammah described a purification process for obtaining purified potassium nitrate from saltpeter. First, the saltpeter is boiled in a small amount of water and then reacted with potassium carbonate from wood ashes. This removes calcium and magnesium salts as precipitates, leaving a potassium nitrate solution. Evaporating the liquid yielded the chemical, which was used to make gunpowder.}}<!-- RS; this author has previously been cited over 60 times on en=WP.--><br>• {{cite web|last=Calvert|first=J. B.|url=http://mysite.du.edu/~jcalvert/phys/chromang.htm|title=Chromium and Manganese|access-date=10 December 2022|date=24 January 2003|url-status=dead|archive-url=https://web.archive.org/web/20161231161307/http://mysite.du.edu/~jcalvert/phys/chromang.htm|archive-date=31 December 2016|trans-quote=See 3rd paragraph under heading "The Metals and Their Properties"}}</ref>{{Failed verification|date=April 2023}}{{Citation needed|date=April 2023}} | ||
| * ], derived from ] ] (in Bergman) | * ] ] (in Bergman) | ||
| * ] ] (in Bergman; previously used for ] of sulfur) | * ] ] (in Bergman; previously used for ] of sulfur) | ||
| * ] ] (in Lavoisier) | * ] ] (in Lavoisier) | ||
| Line 140: | Line 140: | ||
| ===Works cited=== | ===Works cited=== | ||
| * {{cite book|last1=Friedlander|first1=Walter J.|date=1992|title=The Golden Wand of Medicine: A History of the Caduceus Symbol in Medicine|series=Contributions in Medical Studies, 35|location=New York|publisher=Greenwood Press|isbn=0-313-28023-1}} | * {{cite book|last1=Friedlander|first1=Walter J.|date=1992|title=The Golden Wand of Medicine: A History of the Caduceus Symbol in Medicine|series=Contributions in Medical Studies, 35|location=New York|publisher=Greenwood Press|isbn=0-313-28023-1}} | ||
| * {{cite book|last1=Holmyard|first1=Eric J.|author1-link=Eric John Holmyard|date=1957|title=Alchemy |
* {{cite book|last1=Holmyard|first1=Eric J.|author1-link=Eric John Holmyard|date=1957|title=Alchemy|location=Harmondsworth|publisher=Penguin Books|oclc=2080637}} | ||
| * {{cite book|last=Reutter de Rosemont |first=Louis |date= |
* {{cite book|last=Reutter de Rosemont |first=Louis |date=1932 |title=Histoire de la pharmacie a travers les ages |volume=II |pages=4 plates after p 260 and 2 plates after p 268}} | ||
| ==External links== | ==External links== | ||
Revision as of 01:26, 23 April 2023
Symbols used in pre-19th-century chemistry

|

|
| Alchemical symbols before Lavoisier | |
Alchemical symbols, originally devised as part of alchemy, were used to denote some elements and some compounds until the 18th century. Although notation was partly standardized, style and symbol varied between alchemists. Lüdy-Tenger published an inventory of 3,695 symbols and variants, and that was not exhaustive, omitting for example many of the symbols used by Isaac Newton. This page therefore lists only the most common symbols.
Three primes
According to Paracelsus (1493–1541), the three primes or tria prima – of which material substances are immediately composed – are:
- Sulfur or soul, the principle of combustibility: 🜍 (
 )
) - Mercury or spirit, the principle of fusibility and volatility: ☿ (
 )
) - Salt or body, the principle of non-combustibility and non-volatility: 🜔 (
 )
)
Four basic elements
Main article: Classical elementsWestern alchemy makes use of the four classical elements. The symbols used for these are:
Seven planetary metals
Main article: Classical planets in Western alchemy
The seven metals known since Classical times in Europe were associated with the seven classical planets; this figured heavily in alchemical symbolism. The exact correlation varied over time, and in early centuries bronze or electrum were sometimes found instead of mercury, or copper for Mars instead of iron; however, gold, silver, and lead had always been associated with the Sun, Moon, and Saturn. The associations below are attested from the 7th century and had stabilized by the 15th. They started breaking down with the discovery of antimony, bismuth, and zinc in the 16th century. Alchemists would typically call the metals by their planetary names, e.g. "Saturn" for lead, "Mars" for iron; compounds of tin, iron, and silver continued to be called "jovial", "martial", and "lunar"; or "of Jupiter", "of Mars", and "of the moon", through the 17th century. The tradition remains today with the name of the element mercury, where chemists decided the planetary name was preferable to common names like "quicksilver", and in a few archaic terms such as lunar caustic (silver nitrate) and saturnism (lead poisoning).
- Lead, corresponding with Saturn ♄ (
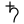 )
) - Tin, corresponding with Jupiter ♃ (
 )
) - Iron, corresponding with Mars ♂ (
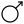 )
) - Gold, corresponding with the Sun ☉ 🜚 ☼ (


 )
) - Copper, corresponding with Venus ♀ (
 )
) - Quicksilver, corresponding with Mercury ☿ (
 )
) - Silver, corresponding with the Moon ☽ or ☾ (
 or
or  )
)
Mundane elements and later metals

- Antimony ♁ (
 ) (in Newton), also
) (in Newton), also 
- Arsenic 🜺 (
 )
) - Bismuth ♆ (
 ) (in Newton), 🜘 (
) (in Newton), 🜘 ( ) (in Bergman)
) (in Bergman) - Cobalt
 (approximately 🜶) (in Bergman)
(approximately 🜶) (in Bergman) - Magnesia (alba), source of later magnesium ⚩ (
 )
) - Manganese
 (in Bergman)
(in Bergman) - Nickel
 (in Bergman; previously used for regulus of sulfur)
(in Bergman; previously used for regulus of sulfur) - Oxygen
 (in Lavoisier)
(in Lavoisier) - Phlogiston
 (in Bergman)
(in Bergman) - Phosphorus
 or
or 
- Platinum
 or
or 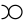 (in Bergman et al.)
(in Bergman et al.) - Sulfur 🜍 (
 ) (in Newton)
) (in Newton) - Zinc
 (in Bergman)
(in Bergman)
Alchemical compounds

The following symbols, among others, have been adopted into Unicode.
- Acid (incl. vinegar) 🜊 (
 )
) - Sal ammoniac (ammonium chloride) 🜹 (
 )
) - Aqua fortis (nitric acid) 🜅 (
 ), A.F.
), A.F. - Aqua regia (nitro-hydrochloric acid) 🜆 (
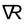 ), 🜇 (
), 🜇 (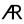 ), A.R.
), A.R. - Spirit of wine (concentrated ethanol; called aqua vitae or spiritus vini) 🜈 (
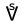 ), S.V. or 🜉 (
), S.V. or 🜉 ( )
) - Amalgam (alloys of a metal and mercury) 🝛 (
 ) = a͞a͞a (one of several abbreviations).
) = a͞a͞a (one of several abbreviations). - Cinnabar (mercury sulfide) 🜓 (
 )
) - Vinegar (distilled) 🜋 (
 ) (in Newton)
) (in Newton) - Vitriol (sulfates) 🜖 (
 )
) - Black sulphur (residue from sublimation of sulfur) 🜏 (
 )
)
Alchemical processes

The alchemical magnum opus was sometimes expressed as a series of chemical operations. In cases where these numbered twelve, each could be assigned one of the Zodiac signs as a form of cryptography. The following example can be found in Pernety's Dictionnaire mytho-hermétique (1758):
- Calcination (Aries
 ) ♈︎
) ♈︎ - Congelation (Taurus
 ) ♉︎
) ♉︎ - Fixation (Gemini
 ) ♊︎
) ♊︎ - Solution (Cancer
 ) ♋︎
) ♋︎ - Digestion (Leo
 ) ♌︎
) ♌︎ - Distillation (Virgo
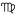 ) ♍︎
) ♍︎ - Sublimation (Libra
 ) ♎︎
) ♎︎ - Separation (Scorpio
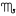 ) ♏︎
) ♏︎ - Ceration (Sagittarius
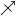 ) ♐︎
) ♐︎ - Fermentation (Capricorn
 ) ♑︎ (Putrefaction)
) ♑︎ (Putrefaction) - Multiplication (Aquarius
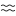 ) ♒︎
) ♒︎ - Projection (Pisces
 ) ♓︎
) ♓︎
Units
Several symbols indicate units of time.
Gallery
A list of symbols published in 1932:
- File:De Rosemont (1932) plate 1.jpg
- File:De Rosemont (1932) plate 2.jpg
- File:De Rosemont (1932) plate 3.jpg
- File:De Rosemont (1932) plate 4.jpg
- File:De Rosemont (1932) plate 5.jpg
- File:De Rosemont (1932) plate 6.jpg
-
(all 6 plates, large file)
Unicode
Main article: Alchemical Symbols (Unicode block)The Alchemical Symbols block was added to Unicode in 2010 as part of Unicode 6.0.
| Alchemical Symbols Official Unicode Consortium code chart (PDF) | ||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | A | B | C | D | E | F | |
| U+1F70x | 🜀 | 🜁 | 🜂 | 🜃 | 🜄 | 🜅 | 🜆 | 🜇 | 🜈 | 🜉 | 🜊 | 🜋 | 🜌 | 🜍 | 🜎 | 🜏 |
| U+1F71x | 🜐 | 🜑 | 🜒 | 🜓 | 🜔 | 🜕 | 🜖 | 🜗 | 🜘 | 🜙 | 🜚 | 🜛 | 🜜 | 🜝 | 🜞 | 🜟 |
| U+1F72x | 🜠 | 🜡 | 🜢 | 🜣 | 🜤 | 🜥 | 🜦 | 🜧 | 🜨 | 🜩 | 🜪 | 🜫 | 🜬 | 🜭 | 🜮 | 🜯 |
| U+1F73x | 🜰 | 🜱 | 🜲 | 🜳 | 🜴 | 🜵 | 🜶 | 🜷 | 🜸 | 🜹 | 🜺 | 🜻 | 🜼 | 🜽 | 🜾 | 🜿 |
| U+1F74x | 🝀 | 🝁 | 🝂 | 🝃 | 🝄 | 🝅 | 🝆 | 🝇 | 🝈 | 🝉 | 🝊 | 🝋 | 🝌 | 🝍 | 🝎 | 🝏 |
| U+1F75x | 🝐 | 🝑 | 🝒 | 🝓 | 🝔 | 🝕 | 🝖 | 🝗 | 🝘 | 🝙 | 🝚 | 🝛 | 🝜 | 🝝 | 🝞 | 🝟 |
| U+1F76x | 🝠 | 🝡 | 🝢 | 🝣 | 🝤 | 🝥 | 🝦 | 🝧 | 🝨 | 🝩 | 🝪 | 🝫 | 🝬 | 🝭 | 🝮 | 🝯 |
| U+1F77x | 🝰 | 🝱 | 🝲 | 🝳 | 🝴 | 🝵 | 🝶 | 🝻 | 🝼 | 🝽 | 🝾 | 🝿 | ||||
Notes
| ||||||||||||||||
See also
Other symbols commonly used in alchemy and related esoteric traditions:
- Astronomical symbols – Symbols in astronomy
- Astrological symbols – Symbols denoting astrological concepts
- Planet symbols – Graphical symbols used in astrology and astronomyPages displaying short descriptions of redirect targets
- Suns in alchemy – Sun symbols have a variety of uses
- Monas Hieroglyphica – 1564 book by John Dee about an esoteric symbol
- Rub el Hizb – Islamic symbol in the shape of an octagram
- Seal of Solomon – Signet ring attributed to the Israelite king Solomon
- Rosy Cross – Western esoteric symbol
- Eye of Providence – SymbolPages displaying short descriptions with no spaces
- Sigil – Magical symbol, as used by Hermetic theurgists
- Sigillum Dei – Seal of God, or Seal of Truth, according to John Dee
Footnotes
- For example, Mercury was tin and Jupiter was electrum in Marcianus.
References
- Fritz Lüdy-Tenger (1928) Alchemistische und chemische Zeichen. Wolfgang Schneider (1962) Lexicon alchemistisch-pharmazeutischer Symbole covers many of the same symbols with a cross-index and indicates synonyms.
- Holmyard 1957, p. 170; cf. Friedlander 1992, pp. 75–76. For the symbols, see Holmyard 1957, p. 149 and Bergman's table as shown above.
- Holmyard 1957, p. 149.
- ^ Crosland, Maurice (2004). Historical Studies in the Language of Chemistry.
- ^ Holmyard 1957, p. 149
- Newman, William R.; Walsh, John A.; Kowalczyk, Stacy; Hooper, Wallace E.; Lopez, Tamara (March 6, 2009). "Proposal for Alchemical Symbols in Unicode" (PDF). Indiana University. p. 11. Unicode: 26A9.
Cf. item 8, "Magnesia" in this chart from Reutter de Rosemont, Louis (1931). Histoire de la pharmacie a travers les ages [History of pharmacy through the ages] (in French). Paris: J. Peyronnet. - The pure metal was not isolated until 1808; the alchemists' substance was the salt "magnesia alba":
• Multhauf, Robert P. (1975-12-09). "A history of magnesia alba". Annals of Science. 33 (1976). Milton Park: Taylor & Francis: 197–200. doi:10.1080/00033797600200231. Retrieved 2023-04-14.By the time of Pliny (first century A.D.) 'magnesian earth' came in several varieties. He mentions five, one of them, called 'magnet', clearly being the celebrated lodestone; the others varied in color from black to white.... As for the material to which the name 'magnesia alba' ultimately became attached, it was a neglected residue of the process conventional in Europe from the 14th century for the production of saltpetre.
• Helmenstine, Anne Marie (2022-03-01). "Saltpeter or Potassium Nitrate Facts". ThoughtCo. Retrieved 2023-04-14.In 1270, Syrian chemist Hasan al-Rammah described a purification process for obtaining purified potassium nitrate from saltpeter. First, the saltpeter is boiled in a small amount of water and then reacted with potassium carbonate from wood ashes. This removes calcium and magnesium salts as precipitates, leaving a potassium nitrate solution. Evaporating the liquid yielded the chemical, which was used to make gunpowder.
• Calvert, J. B. (24 January 2003). "Chromium and Manganese". Archived from the original on 31 December 2016. Retrieved 10 December 2022. [See 3rd paragraph under heading "The Metals and Their Properties"] - Explanation of the Chimical Characters from Nicaise Le Febvre, A compleat body of chymistry, London, 1670.
- See Holmyard 1957, p. 150.
- "Unicode 6.0.0". Unicode Consortium. 11 October 2010. Retrieved 21 October 2019.
Works cited
- Friedlander, Walter J. (1992). The Golden Wand of Medicine: A History of the Caduceus Symbol in Medicine. Contributions in Medical Studies, 35. New York: Greenwood Press. ISBN 0-313-28023-1.
- Holmyard, Eric J. (1957). Alchemy. Harmondsworth: Penguin Books. OCLC 2080637.
- Reutter de Rosemont, Louis (1932). Histoire de la pharmacie a travers les ages. Vol. II. pp. 4 plates after p 260 and 2 plates after p 268.
External links
![]() Media related to Alchemical symbols at Wikimedia Commons
Media related to Alchemical symbols at Wikimedia Commons
- wikt:Appendix:Unicode/Alchemical Symbols
- Alchemical symbols in Unicode 14.0