| Revision as of 16:36, 10 March 2023 editLicenceToCrenellate (talk | contribs)Extended confirmed users, Rollbackers18,206 edits Linting fix← Previous edit | Revision as of 19:31, 12 June 2023 edit undoScyrme (talk | contribs)Extended confirmed users19,963 edits GHS omission ruleNext edit → | ||
| Line 51: | Line 51: | ||
| | GHSPictograms = {{GHS07}}{{GHS09}} | | GHSPictograms = {{GHS07}}{{GHS09}} | ||
| | GHSSignalWord = Warning | | GHSSignalWord = Warning | ||
| | HPhrases = {{H-phrases|302 |
| HPhrases = {{H-phrases|302|410}} | ||
| | PPhrases = {{P-phrases|264|270|273|301+312|330|391|501}} | | PPhrases = {{P-phrases|264|270|273|301+312|330|391|501}} | ||
| }} | }} | ||
Revision as of 19:31, 12 June 2023
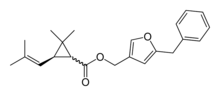 | |
| Names | |
|---|---|
| IUPAC names
(5-benzylfuran-3-yl)methyl (2R)-2,2-dimethyl-3-(2-methylprop-1-en-1-yl)cyclopropane-1-carboxylate; 5-benzyl-3-[({[(3R)-2,2-dimethyl-3-(2-methylprop-1-en-1-yl) cyclopropyl]carbonyl}oxy)methyl]furan; (5-Benzyl-3-furyl)methyl-2,2-dimethyl-3-(2-methyl-1-propen-1-yl)cyclopropancarboxylate; 5-benzyl-3-furylmethyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; 5-benzyl-3-furylmethyl(1RS)-cis-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; 5-benzyl-3-furylmethyl(±)-cis-trans-chrysanthemate | |
| Other names methyl 2,2-dimethyl-3-(2-methyl-1-propen-1-yl)cyclopropanecarboxylate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.030.842 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII |
|
| UN number | 3082 3349 2902 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H26O3 |
| Molar mass | 338.44 g/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H410 |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P391, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Resmethrin is a pyrethroid insecticide with many uses, including control of the adult mosquito population.
The resmethrin molecule has four stereoisomers determined by cis-trans orientation around a carbon triangle and chirality. Technical resmethrin is a mixture of (1R,trans)-, (1R,cis)-, (1S,trans)-, and (1S,cis)- isomers, typically in a ratio of 4:1:4:1. The 1R isomers (both trans and cis) show strong insecticidal activity, while the 1S isomers do not. The (1R,trans)- isomer is also known as bioresmethrin,(+)-trans-resmethrin, or d-trans-resmethrin; although bioresmethrin has been used alone as a pesticide active ingredient, it is not now registered as a separate active ingredient (AI) by the U.S. EPA. The (1R,cis)- isomer is known as cismethrin, but this is also not registered in the U.S. for use alone as a pesticide AI.
Commercial trade names for products that contain resmethrin are: Chrysron, Crossfire, Lethalaire V-26, Pynosect, Raid Flying Insect Killer, Scourge, SPB-1382, Sun-Bugger #4, Synthrin, Syntox, Vectrin, and Whitmire PT-110.
References
- Pesticide Information Profiles, Extension Toxicology Network (EXTOXNET). Resmethrin
External links
- Resmethrin in the Pesticide Properties DataBase (PPDB)
- Resmethrin Technical Fact Sheet - National Pesticide Information Center
- Pyrethrins and Pyrethroids Fact Sheet - National Pesticide Information Center
- Resmethrin Pesticide Information Profile - Extension Toxicology Network
- MSDS for Scourge Formula II
- WHO/FAO DATA SHEETS ON PESTICIDES,No. 83,RESMETHRIN World Health Organization & Food and Agriculture Organization
- Reregistration Eligibility Decision for Resmethrin (2006) - U.S. Environmental Protection Agency