| Revision as of 00:49, 7 March 2023 editBernardirfan (talk | contribs)Extended confirmed users10,991 edits Replacing HTML with {{chem2}}← Previous edit | Latest revision as of 13:48, 28 September 2023 edit undoTrappist the monk (talk | contribs)Administrators479,905 editsm cite repair;Tag: AWB | ||
| Line 29: | Line 29: | ||
| |C=5 | H=6 | N=1 | |C=5 | H=6 | N=1 | ||
| |ConjugateBase = ] | |ConjugateBase = ] | ||
| |pKa = ~5 <ref>{{cite journal|last1=Linnell|first1=Robert|title=Notes – Dissociation Constants of 2-Substituted Pyridines|journal=Journal of Organic Chemistry|volume=25| |
|pKa = ~5 <ref>{{cite journal|last1=Linnell|first1=Robert|title=Notes – Dissociation Constants of 2-Substituted Pyridines|journal=Journal of Organic Chemistry|volume=25|page=290|year=1960|doi=10.1021/jo01072a623|issue=2}}</ref><ref>{{cite journal|last1=Pearson|first1=Ralph G.|title=Rates of Ionization of Pseudo Acids.1V. Steric Effects in the Base-catalyzed Ionization of Nitroethane|last2=Williams|first2=Forrest V.|journal=Journal of the American Chemical Society|volume=75|page=3073|year=1953|doi=10.1021/ja01109a008|issue=13}}</ref> | ||
| }} | }} | ||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| Line 35: | Line 35: | ||
| }} | }} | ||
| '''Pyridinium''' refers to the ] {{chem2|+}}. It is the ] of ]. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.<ref>{{cite journal|title=Fluorinations With Pyridinium Polyhydrogen Fluoride Reagent: 1-Fluoroadamantane| |
'''Pyridinium''' refers to the ] {{chem2|+}}. It is the ] of ]. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.<ref>{{cite journal|title=Fluorinations With Pyridinium Polyhydrogen Fluoride Reagent: 1-Fluoroadamantane|author=George A. Olah |author2=Michael Watkins |journal=Org. Synth.|year=1978|volume=58|page=75|doi=10.15227/orgsyn.058.0075}}</ref> | ||
| As pyridine is often used as an ] in ]s, pyridinium salts are produced in many ]. Its ] are often ] in the organic solvent, so ] of the pyridinium leaving group complex is an indication of the progress of the reaction. | As pyridine is often used as an ] in ]s, pyridinium salts are produced in many ]. Its ] are often ] in the organic solvent, so ] of the pyridinium leaving group complex is an indication of the progress of the reaction. | ||
| Line 43: | Line 43: | ||
| ==''N''-Alkylpyridinium cations== | ==''N''-Alkylpyridinium cations== | ||
| ] is a ''N''-alkylpyridinium cation that occurs widely in life.]] | ] is a ''N''-alkylpyridinium cation that occurs widely in life.]] | ||
| When the acidic proton is replaced by ], the compounds are called ''N''-alkylpyridinium. A simple representative is ] ({{chem2|+}}). These pyridinium intermediates have been used as electrophiles in synthetic organic chemistry to build dearomatized congeners called dihydropyridines, as demonstrated in one example from Smith in 2021.<ref>{{Cite journal|last1=Grigolo|first1=Thiago A.|last2=Subhit|first2=Ariana R.|last3=Smith|first3=Joel M.|date=2021-09-03|title=Regioselective Asymmetric Alkynylation of N-Alkyl Pyridiniums |
When the acidic proton is replaced by ], the compounds are called ''N''-alkylpyridinium. A simple representative is ] ({{chem2|+}}). These pyridinium intermediates have been used as electrophiles in synthetic organic chemistry to build dearomatized congeners called dihydropyridines, as demonstrated in one example from Smith in 2021.<ref>{{Cite journal|last1=Grigolo|first1=Thiago A.|last2=Subhit|first2=Ariana R.|last3=Smith|first3=Joel M.|date=2021-09-03|title=Regioselective Asymmetric Alkynylation of N-Alkyl Pyridiniums|journal=Organic Letters|volume=23|issue=17|pages=6703–6708|doi=10.1021/acs.orglett.1c02276|pmid=34474575 |s2cid=237401193 |issn=1523-7060}}</ref> Earlier, the same research group also delineated the rules surrounding regioselectivities associated with adding nucleophiles to pyridinium electrophiles with varying substituents.<ref>{{Cite journal|last1=Knight|first1=Brian J.|last2=Tolchin|first2=Zachary A.|last3=Smith|first3=Joel M.|date=2021-03-11|title=A predictive model for additions to N-alkyl pyridiniums|url=https://pubs.rsc.org/en/content/articlelanding/2021/cc/d1cc00056j|journal=Chemical Communications|language=en|volume=57|issue=21|pages=2693–2696|doi=10.1039/D1CC00056J|pmid=33595047 |s2cid=231945207 |issn=1364-548X}}</ref> From a commercial perspective, an important pyridinium compound is the ] ].<ref name=Ullmann>{{Ullmann|first1=Shinkichi|last1=Shimizu|first2=Nanao|last2=Watanabe|first3=Toshiaki|last3=Kataoka|first4=Takayuki|last4= Shoji|first5=Nobuyuki|last5=Abe|first6=Sinji|last6=Morishita|first7=Hisao|last7=Ichimura|title=Pyridine and Pyridine Derivatives|year=2007|doi=10.1002/14356007.a22_399}}</ref> | ||
| ==See also== | ==See also== | ||
Latest revision as of 13:48, 28 September 2023
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Pyridin-1-ium | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | [C5H5NH] | ||
| Molar mass | 80.110 g·mol | ||
| Acidity (pKa) | ~5 | ||
| Conjugate base | Pyridine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Pyridinium refers to the cation [C5H5NH]. It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is often used as an organic base in chemical reactions, pyridinium salts are produced in many acid-base reactions. Its salts are often insoluble in the organic solvent, so precipitation of the pyridinium leaving group complex is an indication of the progress of the reaction.
Pyridinium cations are aromatic, as determined through Hückel's rule. They are isoelectronic with benzene.
N-Alkylpyridinium cations
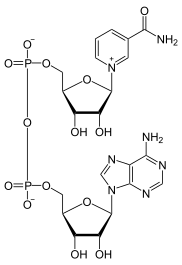
When the acidic proton is replaced by alkyl, the compounds are called N-alkylpyridinium. A simple representative is N-methylpyridinium ([C5H5NCH3]). These pyridinium intermediates have been used as electrophiles in synthetic organic chemistry to build dearomatized congeners called dihydropyridines, as demonstrated in one example from Smith in 2021. Earlier, the same research group also delineated the rules surrounding regioselectivities associated with adding nucleophiles to pyridinium electrophiles with varying substituents. From a commercial perspective, an important pyridinium compound is the herbicide paraquat.
See also
References
- Linnell, Robert (1960). "Notes – Dissociation Constants of 2-Substituted Pyridines". Journal of Organic Chemistry. 25 (2): 290. doi:10.1021/jo01072a623.
- Pearson, Ralph G.; Williams, Forrest V. (1953). "Rates of Ionization of Pseudo Acids.1V. Steric Effects in the Base-catalyzed Ionization of Nitroethane". Journal of the American Chemical Society. 75 (13): 3073. doi:10.1021/ja01109a008.
- George A. Olah; Michael Watkins (1978). "Fluorinations With Pyridinium Polyhydrogen Fluoride Reagent: 1-Fluoroadamantane". Org. Synth. 58: 75. doi:10.15227/orgsyn.058.0075.
- "Aromatic Compounds" (PDF). Alex Roche, Rutgers University.
- Grigolo, Thiago A.; Subhit, Ariana R.; Smith, Joel M. (2021-09-03). "Regioselective Asymmetric Alkynylation of N-Alkyl Pyridiniums". Organic Letters. 23 (17): 6703–6708. doi:10.1021/acs.orglett.1c02276. ISSN 1523-7060. PMID 34474575. S2CID 237401193.
- Knight, Brian J.; Tolchin, Zachary A.; Smith, Joel M. (2021-03-11). "A predictive model for additions to N-alkyl pyridiniums". Chemical Communications. 57 (21): 2693–2696. doi:10.1039/D1CC00056J. ISSN 1364-548X. PMID 33595047. S2CID 231945207.
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399. ISBN 978-3527306732.