|
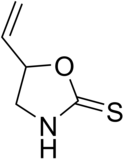
'''Goitrin''' is a sulfur-containing ], Goitrin.pnga cyclic ], that reduces the production of ]s such as ].<ref>{{cite journal |vauthors=McMillan M, Spinks EA, Fenwick GR |title=Preliminary Observations on the Effect of Dietary Brussels Sprouts on Thyroid Function |journal=Hum Toxicol |volume=5 |issue=1 |pages=15–19 |date=January 1986 |pmid=2419242 |doi=10.1177/096032718600500104}}</ref> It is found in cruciferous vegetables such as ], ]s and ],<ref>{{cite journal |vauthors=Lüthy J, Carden B, Friederich U, Bachmann M |title=Goitrin — a nitrosatable constituent of plant foodstuffs |journal=Experientia |volume=40 |issue=5 |pages=452–453 |date=May 1984 |pmid=6723906 |doi=10.1007/BF01952381}}</ref> and is formed by the hydrolysis of a ]: ] or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate (2-hydroxy-3-butenyl isothiocyanate) derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group (allowing a five-membered ring to be formed). Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate (or glucosinolates such as ] and ] which liberate ]) have ]ic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.<ref name=Verhoeven>{{cite journal |vauthors=Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G |title=A review of mechanisms underlying anticarcinogenicity by brassica vegetables |journal=Chem. Biol. Interact. |volume=103 |issue=2 |pages=79–129 |date=February 1997 |pmid=9055870 |doi= 10.1016/S0009-2797(96)03745-3}}</ref> |
|
'''Goitrin''' is an ] classified as a derivative of ] and as a cyclic ]. It reduces the production of ]s such as ].<ref>{{cite journal |vauthors=McMillan M, Spinks EA, Fenwick GR |title=Preliminary Observations on the Effect of Dietary Brussels Sprouts on Thyroid Function |journal=Hum Toxicol |volume=5 |issue=1 |pages=15–19 |date=January 1986 |pmid=2419242 |doi=10.1177/096032718600500104}}</ref> It is found in cruciferous vegetables such as ], ]s and ],<ref>{{cite journal |vauthors=Lüthy J, Carden B, Friederich U, Bachmann M |title=Goitrin — a nitrosatable constituent of plant foodstuffs |journal=Experientia |volume=40 |issue=5 |pages=452–453 |date=May 1984 |pmid=6723906 |doi=10.1007/BF01952381}}</ref> and is formed by the hydrolysis of a ]: ] or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate (2-hydroxy-3-butenyl isothiocyanate) derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group (allowing a five-membered ring to be formed). Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate (or glucosinolates such as ] and ] which liberate ]) have ]ic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.<ref name=Verhoeven>{{cite journal |vauthors=Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G |title=A review of mechanisms underlying anticarcinogenicity by brassica vegetables |journal=Chem. Biol. Interact. |volume=103 |issue=2 |pages=79–129 |date=February 1997 |pmid=9055870 |doi= 10.1016/S0009-2797(96)03745-3}}</ref> |