| Revision as of 23:56, 14 February 2024 edit51.155.110.141 (talk) →Organic synthesis: added an example of use as a dehydrating agent, with source← Previous edit | Latest revision as of 17:52, 31 July 2024 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,690 edits →Organic synthesis: replace patent (not RS) with ref to thiocyanuric acid, and replace white-spacey image with line eq | ||
| Line 88: | Line 88: | ||
| Cyanuric chloride is also used as a precursor to dyes and crosslinking agents. The largest class of these dyes are the sulfonated triazine-stilbene optical brighteners (OBA) or fluorescent whitening agents (FWA) commonly found in detergent formulas and white paper.<ref name=Ullmann/> Many ] also incorporate a triazine ring. They are also manufactured by way of the chloride displacement reaction shown above.<ref name="ashford" /><ref name="TappeHelmling2000">{{cite book|last1=Tappe|first1=Horst|title=Ullmann's Encyclopedia of Industrial Chemistry|last2=Helmling|first2=Walter|last3=Mischke|first3=Peter|last4=Rebsamen|first4=Karl|last5=Reiher|first5=Uwe|last6=Russ|first6=Werner|last7=Schläfer|first7=Ludwig|last8=Vermehren|first8=Petra|year=2000|doi=10.1002/14356007.a22_651|isbn=978-3527306732}}</ref> | Cyanuric chloride is also used as a precursor to dyes and crosslinking agents. The largest class of these dyes are the sulfonated triazine-stilbene optical brighteners (OBA) or fluorescent whitening agents (FWA) commonly found in detergent formulas and white paper.<ref name=Ullmann/> Many ] also incorporate a triazine ring. They are also manufactured by way of the chloride displacement reaction shown above.<ref name="ashford" /><ref name="TappeHelmling2000">{{cite book|last1=Tappe|first1=Horst|title=Ullmann's Encyclopedia of Industrial Chemistry|last2=Helmling|first2=Walter|last3=Mischke|first3=Peter|last4=Rebsamen|first4=Karl|last5=Reiher|first5=Uwe|last6=Russ|first6=Werner|last7=Schläfer|first7=Ludwig|last8=Vermehren|first8=Petra|year=2000|doi=10.1002/14356007.a22_651|isbn=978-3527306732}}</ref> | ||
| ==Reactivity== | |||
| ⚫ | ==Organic synthesis== | ||
| ⚫ | The chloride centers are easily replaced. ]s give ] derivatives, for example in the synthesis of ]s:<ref>{{cite journal | title = Kilogram-Scale Synthesis of a Second-Generation Dendrimer Based on 1,3,5-Triazine Using Green and Industrially Compatible Methods with a Single Chromatographic Step | author = Abdellatif Chouai | author2 = Eric E. Simanek | name-list-style = amp | journal = ] | year = 2008 | volume = 73 | pages = 2357–2366 | doi = 10.1021/jo702462t | pmid = 18307354 | issue = 6| s2cid = 24304872 }}</ref><ref>Reagent: ], amine ]: ]</ref> | ||
| :{{chem2|(ClCN)3 + R2NH -> (R2NCN)3 + 3 HCl }} | |||
| It reacts with ] to give ] ({{chem2|(S\dCNH)3}}).<ref name=Atw>{{cite journal |doi=10.1021/ic0103188 |title=Chemistry of 2,4,6-Trimercapto-1,3,5-triazine (TMT): Acid Dissociation Constants and Group 2 Complexes |date=2001 |last1=Henke |first1=Kevin R. |last2=Hutchison |first2=Aaron R. |last3=Krepps |first3=Matthew K. |last4=Parkin |first4=Sean |last5=Atwood |first5=David A. |journal=Inorganic Chemistry |volume=40 |issue=17 |pages=4443–4447 |pmid=11487353 }}</ref> | |||
| ⚫ | ===Organic synthesis=== | ||
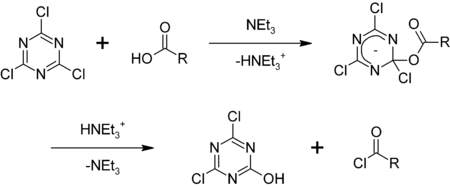
| Cyanuric chloride is employed as a ] in ] for the conversion of alcohols into alkyl chlorides,<ref>{{cite journal | journal = ] | volume = 35 | issue = 11 | pages = 3967-3968 | year = 1970 | doi = 10.1021/jo00836a088 | author = Stanley R. Sandler | title = Cyanuric chloride. A novel laboratory hydrochlorinating reagent for alcohols}}</ref> and carboxylic acids into acyl chlorides:<ref>{{cite journal | journal = ] | volume = 20 | issue = 32 | pages = 3037–3040 | year = 1979 | doi = 10.1016/S0040-4039(00)71006-9 | author = K. Venkataraman | author2 = D. R. Wagle | name-list-style = amp | title = Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides}}</ref> | Cyanuric chloride is employed as a ] in ] for the conversion of alcohols into alkyl chlorides,<ref>{{cite journal | journal = ] | volume = 35 | issue = 11 | pages = 3967-3968 | year = 1970 | doi = 10.1021/jo00836a088 | author = Stanley R. Sandler | title = Cyanuric chloride. A novel laboratory hydrochlorinating reagent for alcohols}}</ref> and carboxylic acids into acyl chlorides:<ref>{{cite journal | journal = ] | volume = 20 | issue = 32 | pages = 3037–3040 | year = 1979 | doi = 10.1016/S0040-4039(00)71006-9 | author = K. Venkataraman | author2 = D. R. Wagle | name-list-style = amp | title = Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides}}</ref> | ||
| :] | :] | ||
| It is also used as a dehydrating agent, e.g. in the conversion of ] to ],<ref>{{cite journal | journal = ] | volume = 8 | pages = 657-658 | year = 1980 | doi = 10.1055/s-1980-29160 | author = George A. Olah | author2 = Subhash C. Narang | author3 = Alexander P. Fung | author4 = B. G. Balaram Gupta | name-list-style = amp | title = Synthetic Methods and Reactions; 82. Cyanuric Chloride, a Mild Dehydrating Agent in the Preparation of Nitriles from Amides}}</ref> and for the activation of carboxylic acids for reduction to alcohols. Heating with ] gives "Gold's reagent" Me<sub>2</sub>NCH=NCH=NMe<sub>2</sub><sup>+</sup>Cl<sup>−</sup>, which is a versatile source of aminoalkylations and a precursor to heterocycles.<ref>Probst, D. A.; Hanson, P. R.; Barda, D. A. "Cyanuric Chloride" in Encyclopedia of Reagents for Organic Synthesis, 2004, John Wiley & Sons. {{doi|10.1002/047084289X.rn00320}}</ref><ref>{{OrgSynth | collvol = 7 | collvolpages = 197 | prep = cv7p0197 | year = 1990 | author = John T. Gupton | author2 = Steven A. Andrews | title = β-Dimethylaminomethylenation: ''N'',''N''-Dimethyl-''N''<nowiki>'</nowiki>-p-tolylformamidine}}</ref> | It is also used as a dehydrating agent, e.g. in the conversion of ] to ],<ref>{{cite journal | journal = ] | volume = 8 | pages = 657-658 | year = 1980 | doi = 10.1055/s-1980-29160 | author = George A. Olah | author2 = Subhash C. Narang | author3 = Alexander P. Fung | author4 = B. G. Balaram Gupta | name-list-style = amp | title = Synthetic Methods and Reactions; 82. Cyanuric Chloride, a Mild Dehydrating Agent in the Preparation of Nitriles from Amides}}</ref> and for the activation of carboxylic acids for reduction to alcohols. Heating with ] gives "Gold's reagent" Me<sub>2</sub>NCH=NCH=NMe<sub>2</sub><sup>+</sup>Cl<sup>−</sup>, which is a versatile source of aminoalkylations and a precursor to heterocycles.<ref>Probst, D. A.; Hanson, P. R.; Barda, D. A. "Cyanuric Chloride" in Encyclopedia of Reagents for Organic Synthesis, 2004, John Wiley & Sons. {{doi|10.1002/047084289X.rn00320}}</ref><ref>{{OrgSynth | collvol = 7 | collvolpages = 197 | prep = cv7p0197 | year = 1990 | author = John T. Gupton | author2 = Steven A. Andrews | title = β-Dimethylaminomethylenation: ''N'',''N''-Dimethyl-''N''<nowiki>'</nowiki>-p-tolylformamidine}}</ref> | ||
| ⚫ | The chloride centers are easily replaced |
||
| :] | |||
| It is also employed the synthesis of an experimental ] ligand.:<ref>{{Ref patent | |||
| |country=WO | |||
| |number=03101980 | |||
| |status= application | |||
| |title=1,3,5-TRIAZINE DERIVATIVES AS LIGANDS FOR HUMAN ADENOSINE-A3 RECEPTORS | |||
| |pubdate=2003-12-11 | |||
| |fdate= | |||
| |pridate= | |||
| |invent1= | |||
| |assign1= | |||
| }} (Reagent number two: ], base ])</ref> | |||
| :] | |||
| Cyanuric chloride can also be used as an alternative to ] in the ].<ref>{{cite journal | last1 = De Luca | first1 = L. | last2 = Giacomelli | first2 = G. | last3 = Procheddu | first3 = A | year = 2001 | title =A Mild and Efficient Alternative to the Classical Swern Oxidation| journal = J. Org. Chem. | volume = 66| issue = 23| pages = 7907–7909 | doi=10.1021/jo015935s| pmid = 11701058 }}</ref> | Cyanuric chloride can also be used as an alternative to ] in the ].<ref>{{cite journal | last1 = De Luca | first1 = L. | last2 = Giacomelli | first2 = G. | last3 = Procheddu | first3 = A | year = 2001 | title =A Mild and Efficient Alternative to the Classical Swern Oxidation| journal = J. Org. Chem. | volume = 66| issue = 23| pages = 7907–7909 | doi=10.1021/jo015935s| pmid = 11701058 }}</ref> | ||
Latest revision as of 17:52, 31 July 2024

| |

| |
| Names | |
|---|---|
| IUPAC name 2,4,6-Trichloro-1,3,5-triazine | |
| Other names
Trichlorotriazine s-Triazine trichloride Cyanuryl chloride TCT | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 124246 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.287 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2670 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3Cl3N3 |
| Molar mass | 184.40 g·mol |
| Appearance | White powder |
| Odor | pungent |
| Density | 1.32 g/cm |
| Melting point | 144–148 °C (291–298 °F; 417–421 K) |
| Boiling point | 192 °C (378 °F; 465 K) |
| Solubility in water | hydrolyzes |
| Solubility in organic solvents | soluble |
| Solubility in THF | 0.34 g/mL |
| Solubility in CHCl3 | 0.17 g/mL |
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H302, H314, H317, H330 |
| Precautionary statements | P260, P261, P264, P270, P271, P272, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P330, P333+P313, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 485 mg/kg (rat, oral) |
| Safety data sheet (SDS) | ICSC 1231 |
| Related compounds | |
| Related triazines | Cyanuric acid Cyanuric fluoride Cyanuric bromide Trichloroisocyanuric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
Production
Cyanuric chloride is prepared in two steps from hydrogen cyanide via the intermediacy of cyanogen chloride, which is trimerized at elevated temperatures over a carbon catalyst:
In 2005, approximately 200,000 tons were produced.
Industrial uses
It is estimated that 70% of cyanuric chloride is used in the preparation of the triazine-class pesticides, especially atrazine. Such reactions rely on the easy displacement of the chloride with nucleophiles such as amines:
- (ClCN)3 + 2 RNH2 → (RNHCN)(ClCN)2 + RNH3Cl
Other triazine herbicides, such as simazine, anilazine and cyromazine are made in an analogous way.
Cyanuric chloride is also used as a precursor to dyes and crosslinking agents. The largest class of these dyes are the sulfonated triazine-stilbene optical brighteners (OBA) or fluorescent whitening agents (FWA) commonly found in detergent formulas and white paper. Many reactive dyes also incorporate a triazine ring. They are also manufactured by way of the chloride displacement reaction shown above.
Reactivity
The chloride centers are easily replaced. Amines give melamine derivatives, for example in the synthesis of dendrimers:
- (ClCN)3 + R2NH → (R2NCN)3 + 3 HCl
It reacts with hydrosulfide to give thiocyanuric acid ((S=CNH)3).
Organic synthesis
Cyanuric chloride is employed as a reagent in organic synthesis for the conversion of alcohols into alkyl chlorides, and carboxylic acids into acyl chlorides:
It is also used as a dehydrating agent, e.g. in the conversion of amides to nitriles, and for the activation of carboxylic acids for reduction to alcohols. Heating with DMF gives "Gold's reagent" Me2NCH=NCH=NMe2Cl, which is a versatile source of aminoalkylations and a precursor to heterocycles.
Cyanuric chloride can also be used as an alternative to oxalyl chloride in the Swern oxidation.
See also
- Thiazyl chloride trimer – structural analogue with sulfur atoms in-place of carbon
References
- Cyanuric chloride at Chemicalland21.com
- ^ Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_191.
- ^ Ashford's Dictionary of Industrial Chemicals, 3rd edition, 2011, pages 2495-8
- Tappe, Horst; Helmling, Walter; Mischke, Peter; Rebsamen, Karl; Reiher, Uwe; Russ, Werner; Schläfer, Ludwig; Vermehren, Petra (2000). Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a22_651. ISBN 978-3527306732.
- Abdellatif Chouai & Eric E. Simanek (2008). "Kilogram-Scale Synthesis of a Second-Generation Dendrimer Based on 1,3,5-Triazine Using Green and Industrially Compatible Methods with a Single Chromatographic Step". J. Org. Chem. 73 (6): 2357–2366. doi:10.1021/jo702462t. PMID 18307354. S2CID 24304872.
- Reagent: DIPEA, amine protective group: BOC
- Henke, Kevin R.; Hutchison, Aaron R.; Krepps, Matthew K.; Parkin, Sean; Atwood, David A. (2001). "Chemistry of 2,4,6-Trimercapto-1,3,5-triazine (TMT): Acid Dissociation Constants and Group 2 Complexes". Inorganic Chemistry. 40 (17): 4443–4447. doi:10.1021/ic0103188. PMID 11487353.
- Stanley R. Sandler (1970). "Cyanuric chloride. A novel laboratory hydrochlorinating reagent for alcohols". J. Org. Chem. 35 (11): 3967–3968. doi:10.1021/jo00836a088.
- K. Venkataraman & D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides". Tetrahedron Lett. 20 (32): 3037–3040. doi:10.1016/S0040-4039(00)71006-9.
- George A. Olah; Subhash C. Narang; Alexander P. Fung & B. G. Balaram Gupta (1980). "Synthetic Methods and Reactions; 82. Cyanuric Chloride, a Mild Dehydrating Agent in the Preparation of Nitriles from Amides". Synthesis. 8: 657–658. doi:10.1055/s-1980-29160.
- Probst, D. A.; Hanson, P. R.; Barda, D. A. "Cyanuric Chloride" in Encyclopedia of Reagents for Organic Synthesis, 2004, John Wiley & Sons. doi:10.1002/047084289X.rn00320
- John T. Gupton; Steven A. Andrews (1990). "β-Dimethylaminomethylenation: N,N-Dimethyl-N'-p-tolylformamidine". Organic Syntheses; Collected Volumes, vol. 7, p. 197.
- De Luca, L.; Giacomelli, G.; Procheddu, A (2001). "A Mild and Efficient Alternative to the Classical Swern Oxidation". J. Org. Chem. 66 (23): 7907–7909. doi:10.1021/jo015935s. PMID 11701058.